Abstract
Freezing of gait (FoG) is a profoundly disruptive gait disturbance in Parkinson’s disease, causing unintended stops while walking. Therapies for FoG reveal modest and transient effects, resulting in a lack of effective treatments. Here we show proof of concept that FoG can be averted using soft robotic apparel that augments hip flexion. The wearable garment uses cable-driven actuators and sensors, generating assistive moments in concert with biological muscles. In this n-of-1 trial with five repeated measurements spanning 6 months, a 73-year-old male with Parkinson’s disease and substantial FoG demonstrated a robust response to robotic apparel. With assistance, FoG was instantaneously eliminated during indoor walking (0% versus 39 ± 16% time spent freezing when unassisted), accompanied by 49 ± 11 m (+55%) farther walking compared to unassisted walking, faster speeds (+0.18 m s−1) and improved gait quality (−25% in gait variability). FoG-targeting effects were repeatable across multiple days, provoking conditions and environment contexts, demonstrating potential for community use. This study demonstrated that FoG was averted using soft robotic apparel in an individual with Parkinson’s disease, serving as an impetus for technological advancements in response to this serious yet unmet need.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All study data necessary to interpret, verify and extend this work are available in the Source Data section. This includes data for Figs. 1–5 and Extended Data Figs. 2–5. Source data are provided with this paper.
References
Dorsey, E. R. et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953 (2018).
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 16, 877–897 (2017).
Maserejian, N., Vinikoor-Imler, L. & Dilley, A. Estimation of the 2020 global population of Parkinson’s disease (PD). MDS Virtual Congress 2020. https://www.mdsabstracts.org/abstract/estimation-of-the-2020-global-population-of-parkinsons-disease-pd/ (2020).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Wu, T., Hallett, M. & Chan, P. Motor automaticity in Parkinson’s disease. Neurobiol. Dis. 82, 226–234 (2015).
Giladi, N. et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology 56, 1712–1721 (2001).
Nutt, J. G. et al. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 10, 734–744 (2011).
Nonnekes, J. et al. Freezing of gait: a practical approach to management. Lancet Neurol. 14, 768–778 (2015).
Perez-Lloret, S. et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. 71, 884–890 (2014).
Bohnen, N. I. et al. Discussion of research priorities for gait disorders in Parkinson’s disease. Mov. Disord. 37, 253–263 (2022).
Cui, C. K. & Lewis, S. J. G. Future therapeutic strategies for freezing of gait in Parkinson’s disease. Front. Hum. Neurosci. 15, 741918 (2021).
Jenner, P. Treatment of the later stages of Parkinson’s disease—pharmacological approaches now and in the future. Transl. Neurodegener. 4, 3 (2015).
Schaafsma, J. D. et al. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur. J. Neurol. 10, 391–398 (2003).
Davis, J. T., Lyons, K. E. & Pahwa, R. Freezing of gait after bilateral subthalamic nucleus stimulation for Parkinson’s disease. Clin. Neurol. Neurosurg. 108, 461–464 (2006).
Schlenstedt, C. et al. Effect of high-frequency subthalamic neurostimulation on gait and freezing of gait in Parkinson’s disease: a systematic review and meta-analysis. Eur. J. Neurol. 24, 18–26 (2017).
Razmkon, A., Abdollahifard, S., Taherifard, E., Roshanshad, A. & Shahrivar, K. Effect of deep brain stimulation on freezing of gait in patients with Parkinson’s disease: a systematic review. Br. J. Neurosurg. 37, 3–11 (2023).
Winfree, K. N. et al. The effect of step-synchronized vibration on patients with Parkinson’s disease: case studies on subjects with freezing of gait or an implanted deep brain stimulator. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 806–811 (2013).
Nieuwboer, A. Cueing for freezing of gait in patients with Parkinson’s disease: a rehabilitation perspective. Mov. Disord. 23, S475–S481 (2008).
Ginis, P., Nackaerts, E., Nieuwboer, A. & Heremans, E. Cueing for people with Parkinson’s disease with freezing of gait: a narrative review of the state-of-the-art and novel perspectives. Ann. Phys. Rehabil. Med. 61, 407–413 (2018).
Bekkers, E. M. J. et al. Balancing between the two: are freezing of gait and postural instability in Parkinson’s disease connected? Neurosci. Biobehav. Rev. 94, 113–125 (2018).
Nieuwboer, A. & Giladi, N. Characterizing freezing of gait in Parkinson’s disease: models of an episodic phenomenon. Mov. Disord. 28, 1509–1519 (2013).
Rahman, S., Griffin, H. J., Quinn, N. P. & Jahanshahi, M. The factors that induce or overcome freezing of gait in Parkinson’s disease. Behav. Neurol. 19, 127–136 (2008).
Chee, R., Murphy, A., Danoudis, M., Georgiou-Karistianis, N. & Iansek, R. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain 132, 2151–2160 (2009).
Cupertino, L. et al. Biomechanical aspects that precede freezing episode during gait in individuals with Parkinson’s disease: a systematic review. Gait Posture 91, 149–154 (2022).
Nieuwboer, A. et al. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov. Disord. 16, 1066–1075 (2001).
Nieuwboer, A. et al. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain 127, 1650–1660 (2004).
Nieuwboer, A., Chavret, F., Willems, A.-M. & Desloovere, K. Does freezing in Parkinson’s disease change limb coordination? J. Neurol. 254, 1268–1277 (2007).
Albani, G. et al. ‘Masters and servants’ in parkinsonian gait: a three-dimensional analysis of biomechanical changes sensitive to disease progression. Funct. Neurol. 29, 99–105 (2014).
Hausdorff, J. M. et al. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp. Brain Res. 149, 187–194 (2003).
Plotnik, M., Giladi, N. & Hausdorff, J. M. Is freezing of gait in Parkinson’s disease a result of multiple gait impairments? implications for treatment. Parkinsons Dis. 2012, 459321 (2012).
Awad, L. N. et al. A soft robotic exosuit improves walking in patients after stroke. Sci. Transl. Med. 9, eaai9084 (2017).
Esquenazi, A., Talaty, M., Packel, A. & Saulino, M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 91, 911–921 (2012).
Jayaraman, A. et al. Stride management assist exoskeleton vs functional gait training in stroke: a randomized trial. Neurology 92, e263–e273 (2019).
Lerner, Z. F., Damiano, D. L., Park, H.-S., Gravunder, A. J. & Bulea, T. C. A robotic exoskeleton for treatment of crouch gait in children with cerebral palsy: design and initial application. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 650–659 (2017).
Barthel, C., Mallia, E., Debû, B., Bloem, B. R. & Ferraye, M. U. The practicalities of assessing freezing of gait. J. Parkinsons Dis. 6, 667–674 (2016).
Snijders, A. H., Haaxma, C. A., Hagen, Y. J., Munneke, M. & Bloem, B. R. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat. Disord. 18, 149–154 (2012).
Besharat, A. et al. Virtual reality doorway and hallway environments alter gait kinematics in people with Parkinson disease and freezing. Gait Posture 92, 442–448 (2022).
Bloem, B. R., Monje, M. H. G. & Obeso, J. A. Understanding motor control in health and disease: classic single (n = 1) observations. Exp. Brain Res. 238, 1593–1600 (2020).
Dobkin, B. H. Progressive staging of pilot studies to improve phase III trials for motor interventions. Neurorehabil. Neural Repair 23, 197–206 (2009).
Kim, J. et al. Reducing the energy cost of walking with low assistance levels through optimized hip flexion assistance from a soft exosuit. Sci. Rep. 12, 11004 (2022).
Morris, T. R. et al. A comparison of clinical and objective measures of freezing of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 18, 572–577 (2012).
Lewis, S. et al. Stepping up to meet the challenge of freezing of gait in Parkinson’s disease. Transl. Neurodegener. 11, 23 (2022).
Delval, A., Tard, C., Rambour, M., Defebvre, L. & Moreau, C. Characterization and quantification of freezing of gait in Parkinson’s disease: can detection algorithms replace clinical expert opinion? Neurophysiol. Clin. 45, 305–313 (2015).
Gilat, M. How to annotate freezing of gait from video: a standardized method using open-source software. J. Parkinsons Dis. 9, 821–824 (2019).
Delval, A. et al. Objective detection of subtle freezing of gait episodes in Parkinson’s disease. Mov. Disord. 25, 1684–1693 (2010).
Hausdorff, J. M., Balash, Y. & Giladi, N. Time series analysis of leg movements during freezing of gait in Parkinson’s disease: akinesia, rhyme or reason? Physica A 321, 565–570 (2003).
Moore, S. T., MacDougall, H. G. & Ondo, W. G. Ambulatory monitoring of freezing of gait in Parkinson’s disease. J. Neurosci. Methods 167, 340–348 (2008).
Mancini, M. et al. Measuring freezing of gait during daily-life: an open-source, wearable sensors approach. J. Neuroeng. Rehabil. 18, 1 (2021).
Connelly, D. M., Thomas, B. K., Cliffe, S. J., Perry, W. M. & Smith, R. E. Clinical utility of the 2-minute walk test for older adults living in long-term care. Physiother. Can. 61, 78–87 (2009).
Gijbels, D. et al. Predicting habitual walking performance in multiple sclerosis: relevance of capacity and self-report measures. Mult. Scler. 16, 618–626 (2010).
Perera, S., Mody, S. H., Woodman, R. C. & Studenski, S. A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 54, 743–749 (2006).
Vette, A. H. et al. The utility of normative foot floor angle data in assessing toe-walking. Foot 37, 65–70 (2018).
Mariani, B. et al. 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 43, 2999–3006 (2010).
Steffen, T. & Seney, M. Test–retest reliability and minimal detectable change on balance and ambulation tests, the 36-Item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Phys. Ther. 88, 733–746 (2008).
Arens, P. et al. Real-time gait metric estimation for everyday gait training with wearable devices in people poststroke. Wearable Technol. 2, e2 (2021).
Iansek, R., Huxham, F. & McGinley, J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov. Disord. 21, 1419–1424 (2006).
Mancini, M. et al. Clinical and methodological challenges for assessing freezing of gait: future perspectives. Mov. Disord. 34, 783–790 (2019).
Borzì, L. et al. Prediction of freezing of gait in Parkinson’s disease using wearables and machine learning. Sensors 21, 614 (2021).
Prado, A., Kwei, S. K., Vanegas-Arroyave, N. & Agrawal, S. K. Continuous identification of freezing of gait in Parkinson’s patients using artificial neural networks and instrumented shoes. IEEE Trans. Med. Robot. Bionics 3, 554–562 (2021).
Martelli, D. et al. Adaptation of stability during perturbed walking in Parkinson’s disease. Sci. Rep. 7, 17875 (2017).
Lo, A. C. et al. Reduction of freezing of gait in Parkinson’s disease by repetitive robot-assisted treadmill training: a pilot study. J. Neuroeng. Rehabil. 7, 51 (2010).
Barbe, M. T., Cepuran, F., Amarell, M., Schoenau, E. & Timmermann, L. Long-term effect of robot-assisted treadmill walking reduces freezing of gait in Parkinson’s disease patients: a pilot study. J. Neurol. 260, 296–298 (2013).
Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression, and mortality. Neurology 17, 427 (1967).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Powell, L. E. & Myers, A. M. The Activities-specific Balance Confidence (ABC) Scale. J. Gerontol. A 50A, M28–M34 (1995).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Nieuwboer, A. et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 30, 459–463 (2009).
Tosserams, A., Mazaheri, M., Vart, P., Bloem, B. R. & Nonnekes, J. Sex and freezing of gait in Parkinson’s disease: a systematic review and meta-analysis. J. Neurol. 268, 125–132 (2020).
Ricker, J. H. & Axelrod, B. N. Analysis of an oral paradigm for the Trail Making Test. Assessment 1, 47–52 (1994).
Kim, J. et al. Reducing the metabolic rate of walking and running with a versatile, portable exosuit. Science 365, 668–672 (2019).
Sánchez, N., Park, S. & Finley, J. M. Evidence of energetic optimization during adaptation differs for metabolic, mechanical, and perceptual estimates of energetic cost. Sci. Rep. 7, 7682 (2017).
O’Day, J. et al. The turning and barrier course reveals gait parameters for detecting freezing of gait and measuring the efficacy of deep brain stimulation. PLoS ONE 15, e0231984 (2020).
Kratochwill, T. R. Single Subject Research: Strategies for Evaluating Change (Academic Press, 1978).
Bates, B. T. Single-subject methodology: an alternative approach. Med. Sci. Sports Exerc. 28, 631–638 (1996).
Westfall, P. H. & Young, S. S. Resampling-Based Multiple Testing: Examples and Methods for P-value Adjustment (Wiley, 1993).
Acknowledgements
We thank T. Akbas, S. Park, A. Eckert-Erdheim, D. Orzel, A. Huang and S. Sullivan for their contributions to this work. This material is based on work supported by the National Science Foundation (CMMI-1925085; C.J.W.), the National Institutes of Health (U01 TR002775; C.J.W. and T.D.E.) and the Massachusetts Technology Collaborative, Collaborative Research and Development Matching Grant (C.J.W.). This work is also partially funded by the John A. Paulson School of Engineering and Applied Sciences at Harvard University (C.J.W.). J.K. appreciates financial support from the Samsung Scholarship.
Author information
Authors and Affiliations
Contributions
J.K., F.P., T.D.E. and C.J.W. conceived of the study concept and designed the research. J.K. implemented the control and hardware of the robotic apparel. J.K., H.D.Y., N.W., T.B. and A.C. conducted the experiments. J.K. processed and analyzed the experimental data. J.K., F.P., T.D.E. and C.J.W. prepared and revised the paper. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Patents describing the robotic apparel components documented in this article have been filed with the US Patent Office by Harvard University. C.J.W. is an inventor on the following patents and patent applications: US 9,351,900, US 10,278,883, US 14/660,704, US 15/097,744 and US 14/893,934. Harvard University has entered into a licensing agreement with ReWalk Robotics. C.J.W. was previously a paid consultant for ReWalk Robotics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Alice Nieuwboer, Gregory Sawicki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
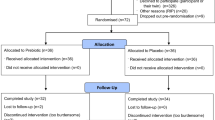
Extended Data Fig. 1 Study flow and protocol overview.
Summary schematic of five study visits. For each study visit, timed walking trials under single-task conditions during medication on-phase were performed (middle column in Study Visits 1-5). Additionally, conditions that further provoked FoG were administered based on medication timing (Study Visit 2), cognitive loading using dual-task challenge (Study Visit 3), and outdoor walking (Study Visit 5). The study also included conditions examining the immediacy of device effects through intermittent assistance (Study Visits 2 and 4), the underlying biomechanical mechanisms (Study Visit 1), and the effects of force levels when providing low assistance (Study Visit 3).
Extended Data Fig. 2 Effects of force levels.
a, Occurrence of freezing during timed 2-minute walking trials. b, Walking distance during timed 2-minute walking trials. All trials were conducted in the laboratory during the medication on-phase and under single-task conditions (Study Visit 3). A peak force of 80 N was applied during ASSIST ON, while a peak force of 20 N was applied during Low ASSIST. Data for ASSIST OFF and ASSIST ON were previously included in Fig. 2.
Extended Data Fig. 3 Intermittent assistance without verbal prompts.
a, Time series data of applied hip flexion force (top) and participant’s stride length (bottom). Sequential intervals of 30-s bouts with and without assistance were implemented across three intervals amounting to a total of 3 min of walking. Different from Fig. 3, the operator did not give any verbal notice of the assistance mode change to the participant. Data during ASSIST ON and ASSIST OFF are plotted in red and gray, respectively, and the gray shaded regions indicate FoG episodes during ASSIST OFF. There was no FoG episode during ASSIST ON. b, Stride length per interval. Stride lengths in all intervals are presented in box plots (center line: median; box limits: upper and lower quartiles; whiskers: 1.5 × interquartile range; points: outliers), and asterisks indicate statistically significant differences (two-sided randomization test with a stride-level median as a test statistic; ***P < 0.001). Interval 1 (n=26 independent strides for ASSIST ON and n=32 independent strides for ASSIST OFF; P < 0.001), interval 2 (n=29 independent strides for ASSIST ON and n=31 independent strides for ASSIST OFF; P < 0.001), and interval 3 (n=26 independent strides for ASSIST ON and n=19 independent strides for ASSIST OFF; P < 0.001). c, Occurrence of freezing per interval based on duration (left y axis) and percent time spent freezing (right y axis).
Extended Data Fig. 4 Preserved regulation of stride length: A potential reason for the effects of the robotic apparel.
Stride length over time during timed 2-minute walking trials. The linear regression slope of stride length leading to the onset of FoG was examined. a, ASSIST OFF vs. ASSIST ON (Study Visit 1). Markers in light gray and red are for ASSIST OFF and ASSIST ON, respectively. The shaded regions in light gray indicate FoG episodes during ASSIST OFF (Linear regression: y = −4.65⋅10−3 × time + 1.15, n = 48 independent strides; a two-sided, one-sample t-test for the slope, P = 3.08⋅10−12, t = −9.37, df = 46). There was no FoG episode during ASSIST ON. b, NO SUIT vs. ASSIST ON (Study Visit 4). Markers in dark gray and red are for NO SUIT and ASSIST ON, respectively. The shaded regions in dark gray indicate FoG episodes during NO SUIT (Linear regression: y = −3.42⋅10−3 × time + 1.09, n = 66 independent strides; a two-sided, one-sample t-test for the slope, P = 2.45⋅10−16, t = −10.97, df = 64). There was no FoG episode during ASSIST ON.
Extended Data Fig. 5 Variability of stride length: A potential reason for the effects of the robotic apparel.
Stride length arrhythmicity during timed 2-minute walking trials (n = 6 independent walking bouts for BASELINE and n = 6 independent walking bouts for ASSIST ON; P = 0.031). Each study visit was conducted on a separate day. A summary bar plot is presented as mean ± s.d., with an asterisk indicating a statistically significant difference (two-sided Wilcoxon signed-rank test; *P < 0.05). Baseline includes both NO SUIT and ASSIST OFF conditions. The coefficient of variance (in stride length) was measured as the ratio of the standard deviation to the mean.
Supplementary information
Supplementary Video 1
The effects of robotic apparel during medication on-phase. Video excerpt during a 2MWT provides a side-by-side comparison of walking with (right; ASSIST ON) and without (left; ASSIST OFF) the robotic apparel, tested during medication on-phase under single-task conditions. A physical therapist provided close supervision for general participant safety during the walking test. Other researchers managed video recording, measured distance with a handheld measuring wheel and ensured having a wheelchair on standby, if needed. Distance counters on the top right and left corners provide walking distance in real time.
Supplementary Video 2
The effects of robotic apparel during medication relative off-phase. Video excerpt during a 2MWT provides a side-by-side comparison of the effects of robotic apparel, tested during suboptimal timing of dopaminergic medication (relative off-phase) under single-task conditions. Experimental setup is identical to Supplementary Video 1.
Supplementary Video 3
Intermittent assistance of the robotic apparel. Video excerpt during a 4-min walking trial demonstrates the immediate effects of the robotic apparel on averting FoG tested during medication on-phase and under single-task conditions. The immediate effects of the robotic apparel were demonstrated by intermittent assistance of the robotic apparel (that is, serial on and off at 30-s intervals). The inset figures on the top-right corner show the applied hip flexion force and the participant’s thigh angle in real time.
Supplementary Video 4
The effects of robotic apparel when walking outdoors. The video excerpt during a 6MWT provides a side-by-side comparison of walking with (right; ASSIST ON) and without (left; NO SUIT) the robotic apparel in real-world, outdoor community settings. The testing took place during the medication on-phase under single-task conditions.
Supplementary Video 5
Effects of the robotic apparel during short-distance walking. Video samples during short-distance walking based on the 10-m walk test (comfortable speed) provide side-by-side comparison of gait quality related to the assistance of robotic apparel. Procedures were performed during medication on-phase and under single-task conditions. Kinematic measurements of walking were obtained using a three-dimensional motion capture system and wearable sensors.
Source data
Source Data Figs. 1–5
Statistical source data for Figs. 1–5.
Source Data Extended Data Figs. 2–5
Statistical source data for Extended Data Figs. 2–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J., Porciuncula, F., Yang, H.D. et al. Soft robotic apparel to avert freezing of gait in Parkinson’s disease. Nat Med 30, 177–185 (2024). https://doi.org/10.1038/s41591-023-02731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02731-8
This article is cited by
-
Soft robotic apparel improves walking in Parkinson’s disease
Nature Reviews Bioengineering (2024)