Abstract
Exoskeletons can augment the performance of unimpaired users and restore movement in individuals with gait impairments. Knowledge of how users interact with wearable devices and of the physiology of locomotion have informed the design of rigid and soft exoskeletons that can specifically target a single joint or a single activity. In this Review, we highlight the main advances of the past two decades in exoskeleton technology and in the development of lower-extremity exoskeletons for locomotor assistance, discuss research needs for such wearable robots and the clinical requirements for exoskeleton-assisted gait rehabilitation, and outline the main clinical challenges and opportunities for exoskeleton technology.
Similar content being viewed by others
Main
Exoskeletons aiding locomotion entered the popular imagination over a century ago, followed by a series of early patents and prototypes1,2,3,4,5. Among other advances in the late twentieth and early twenty-first century, funding from the Exoskeleton for Human Performance Augmentation Program6 of the Defense Advanced Research Projects Agency (DARPA) of the United States enabled the development of wearable robotic devices for the lower extremities (in particular, the Berkeley lower-extremity exoskeleton, BLEEX7,8; the Sarcos Guardian XO9; and the MIT quasi-passive exoskeleton10,11,12,13) to augment strength and reduce effort during the carriage of load. By taking load off the wearer and providing assistive joint torques during walking, these weight-bearing lower-extremity exoskeletons sought to increase load capacity, improve efficiency and endurance, and reduce the perceived difficulty of walking11, potentially benefitting military personnel, first responders and weekend warriors. Measuring the metabolic cost of exoskeleton-aided locomotion became the gold standard for quantifying wearer effort and exertion. As such, demonstrating metabolic cost reductions compared to walking without the robot became a major goal for exoskeletons designed to augment strength and performance. Despite their promise, lower-extremity exoskeletons for performance augmentation in unimpaired users initially failed to demonstrate metabolic cost reductions compared with walking without the exoskeletons14,15,16. Therefore, lighter-weight hardware and autonomous systems were developed and tested in goal-driven experiments, with single-joint systems tested to explore underlying biological mechanisms. These technological advances ultimately enabled exoskeletons to meet the envisioned objectives of reducing the metabolic cost during loaded or unloaded locomotion for military, industrial and recreational applications.
Alongside the development of exoskeletons to enhance the performance of load carriage, devices (in particular, the Lokomat17, Gait Trainer18, lower extremity powered exoskeleton (LOPES)19, active leg exoskeleton (ALEX)20,21 and Rutgers Ankle22) were designed to mechanize physical therapy for individuals re-learning to walk following spinal-cord injury (SCI) or stroke. Common clinical practice calls for physical therapists to manually move the feet and legs of non-ambulatory patients through the motions of walking to facilitate re-learning of movement patterns for functional improvement23. Robotic devices were developed to offload this burden from physical therapists and to improve patient outcomes by delivering precise interventions and training at optimal intensities, unconstrained by the limits of manual assistance. Exoskeletons initially failed to show clinical improvements that would justify their cost compared to traditional non-robotic gait-training methods24,25,26,27,28,29,30. However, a better understanding of the underlying biomechanics and physiology led to an improvement in exoskeleton design, including biologically inspired strategies for actuation and control31,32,33,34,35,36. For example, ‘patient-cooperative control’ has been implemented in devices for gait rehabilitation to allow for more individualized assistance37,38,39,40,41,42,43. Such ‘assist as needed’ approaches are particularly important for augmenting the gait of individuals with some residual walking function while encouraging their own contributions.
In this Review, we first synthesize key advances in exoskeleton technology in the early twenty-first century and highlight recent exoskeleton designs, offering clinical insight into how exoskeletons can improve gait rehabilitation and identifying future directions for wearable robots. For devices designed for unimpaired adults, we focus on gait and exclude research in industrial applications (unless they are particularly relevant). We then discuss exoskeletons aimed at reducing the metabolic cost of walking in unimpaired individuals, which has been a principal focus since the 1990s. For devices intended to be used for people with gait impairments, we exclude research reported only in animal models. Rather, we include representative studies of people with stroke, traumatic brain injury (TBI), multiple sclerosis (MS), cerebral palsy (CP), and complete and incomplete spinal-cord injury (SCI-c, SCI-i). These conditions represent a range of gait impairments that require anywhere from full assistance (the case of SCI) to partial assistance (the case of stroke, MS, TBI and CP).
Wearable robots
The Cybathlon—a non-profit project of the Swiss Federal Institute of Technology in Zürich, in which people with physical disabilities compete against each other every 4 years to complete everyday tasks using assistive technologies—has showcased a variety of exoskeletons used in various challenges44. Lower-extremity exoskeletons are similar to one another in their ability to augment human performance or restore movement; however, they differ in some key characteristics, such as device function and purpose.
Exoskeletons can be broadly classified as weight-bearing devices that transfer load directly to the ground or as joint-targeting devices that augment biological torque at a specific joint or joints to achieve a physiological goal. Weight-bearing and joint-targeting exoskeletons can either be applied for performance augmentation in unimpaired users or for movement restoration in the clinic for people with disabilities (Fig. 1). These exoskeletons can further be designed with different power sources. Autonomous devices require the user to carry a battery to power conventional actuators (which increases their weight). In research-focused systems, the power source and actuators can be placed on a freestanding offboard structure (tethering the wearer to a treadmill). Alternatively, passive designs, in which energy is collected and returned during the gait cycle, do not require any power source45. System-level advances in both exoskeleton types have led to their increased commercial presence, to informative clinical investigations and to a better understanding of how exoskeletons influence biological processes46,47,48,49,50,51.
Weight-bearing exoskeletons
Weight-bearing exoskeletons typically span the entire lower extremity and are made of rigid robotic components that enable multijoint assistance and direct transfer of load to the ground. Such devices were initially designed to reduce metabolic cost in unimpaired individuals during walking. However, to transfer loads to the ground, these devices usually inhibit coordination between the device and the wearer, which causes a change in the wearer’s gait pattern (compared with their optimal pattern), limiting the ability of autonomous weight-bearing devices to lower the metabolic cost of walking14. Therefore, weight-bearing exoskeleton technologies have focused on increasing force production during non-ambulatory tasks, for example to enable workers to lift heavy loads for extended periods of time (FORTIS, Guardian XO, Hanyang Exoskeleton Assistive Robot (HEXAR)-CR5052, Body Extender53,54,55).
Autonomous weight-bearing exoskeletons can also be designed with high power to restore some degree of mobility in people with substantial walking impairments. Several such devices have achieved regulatory approval56 (in particular, Hybrid Limb Assist (HAL)57,58 in Japan and Europe, and ReWalk59, EksoNR60,61 and Indego62 in the United States). Originally designed to support people with paraplegia, these devices can also be applied as gait-training tools for individuals with residual walking capacity49. A variety of such weight-bearing exoskeletons are commercially available (the Atlas, the ExoAtlet, the Hank, the Mina, the SuitX, the ExoH2 and the Twiice).
These devices can further be designed with various features; for example, extended sensor modalities can be integrated in control loops to measure reaction forces between the wearer and the ground58,63, the centre-of-mass position64, or brain activity65,66. Such features may enable the development of weight-bearing exoskeletons with well-coordinated control, allowing the wearer to adapt to new environments with the ease of an unimpaired walker, rather than providing only predefined motions. However, their weight and size limit their capacity to achieve specific physiological goals, such as reducing metabolic cost, because the exoskeleton spans the entire lower extremity and assists multiple joints. Therefore, research efforts have been targeted towards the design of lightweight systems that, instead of transferring load directly to the ground, apply torque in conjunction with biological mechanisms to assist a single joint.
Joint-targeting devices
Joint-targeting devices assist a specific part of the body, enabling insights into how distinct biological features respond to changes in targeted exoskeleton assistance, which cannot be achieved with weight-bearing devices. The physiological effects of joint-targeting exoskeletons were first investigated using an ankle system driven by artificial pneumatic muscles31,32 with an offboard air compressor. By delivering assistance proportional to soleus surface electromyography (EMG), this device not only assisted gait, but also allowed fundamental investigations of the ankle’s function, in particular how passive elements in the ankle muscle–tendon contribute to the efficiency of walking15,34. Knowledge gained from these fundamental experiments greatly contributed to the design of joint-targeting devices that can decrease the metabolic cost of walking in unimpaired individuals with powered67,68,69,70 and passive45 ankle assistance (Fig. 2). Joint-targeting devices for ankle assistance also contributed to our understanding of gait rehabilitation in people with neurological impairments71,72,73,74,75.
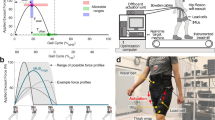
Key developments in exoskeletons for gait assistance from 2000 to present. Green boxes and illustrations denote weight-bearing exoskeletons meant for unimpaired populations, yellow denotes weight-bearing exoskeletons for clinical applications, blue denotes joint-targeting devices for unimpaired populations, and pink denotes joint-targeting devices for clinical populations.
Offboard actuation holds an important role in investigating the responses to joint-targeted assistance to inform the design of autonomous exoskeletons. By eliminating the need for body-worn power supplies and actuators, offboard systems enable heavier and more powerful actuators to serve across multiple experiments76,77,78,79,80,81, making them ideal research systems for rapid prototyping of joint-targeting exoskeletons. The field of wearable robotics has greatly benefitted from offboard actuation, which has allowed the evaluation of wearer sensitivity to varying levels of augmentation power82, the comparison of power-inspired assistance versus moment-inspired assistance83,84, the investigation of the impact of the stiffness of elastic ankle exoskeletons, and the optimization of assistance based on real-time measurements of metabolic cost78,85. Furthermore, offboard actuation has allowed one device to emulate both rigid-link and non-rigid-link exoskeletons for gait training post-stroke86. Additionally, a commercial offboard system (Caplex) can emulate the evaluation of assistive strategies in real time77.
The mechanical designs and control strategies of joint-targeting exoskeletons with offboard actuation can be easily modified to explore the different physiological impacts of the designs; however, these systems are restricted to treadmill-based studies, which may not reflect typical features of walking (such as changes in speed, direction or terrain). By contrast, autonomous systems allow the wearer to freely navigate unconstrained environments. The aim of joint-targeting exoskeletons is not to bear weight, hence autonomous devices do not require large rigid structures. However, rigid components are beneficial for efficiently transferring torques to the body; for example, rigid components and a distal actuator at the ankle decrease the metabolic cost of loaded and unloaded walking in unimpaired individuals69,70,87. Rigid devices have also been developed for assisting other joints, in particular the hip88,89,90,91 and the knee92,93, and for multijoint assistance94 (for example, the Cyberlegs project95). Moreover, rigid joint-targeting autonomous exoskeletons have been commercially developed for industrial, recreational and clinical applications, such as the Stride Management Assist (SMA)96,97,98 and the Gait Enhancing and Motivation System (GEMS)99,100. These autonomous bidirectional hip exoskeletons have been used to investigate new control schemes101,102,103, the augmentation of performance in unimpaired individuals104,105,106, the restoration of gait function in elderly adults107,108, improvements in gait and other mobility in people with CP109,110,111 and clinical effects post-stroke112,113.
Joint-targeting exoskeletons often feature creative designs to mitigate the high energetic cost of distal mass; for example, lightweight textile-based components can be applied to interface with the body and minimize kinematic interference. These soft exoskeletons (often called exosuits) sacrifice some efficiency in transmitting power to the user in exchange for light and minimally restrictive systems, which may be more comfortable to wear for prolonged periods compared with traditionally rigid exoskeletons. Soft exosuits can augment metabolic performance114,115,116,117,118 and enable post-stroke gait assistance at the ankle119,120,121. They have been commercialized (one example is the ReStore122). The textile-based Myosuit assists hip and knee extension with a single actuator, as well as hip flexion with a passive spring-like element during sit-to-stand transitions123 and walking124. A soft wearable robotic ankle-foot orthosis with a bidirectional, tendon-driven and distally mounted actuator can assist plantarflexion and dorsiflexion125. A textile-based device with a ‘cross-wire’ design can be applied at the hip to induce turning126. The XoSoft delivers similar amounts of mechanical power to the ankle and hip as other devices127 but has not yet been evaluated in the clinic or in terms of metabolic cost reduction in unimpaired individuals.
To further reduce costly distal mass, designs based on biological mechanisms are being explored. The human body compensates for energy cost with passive structures such as tendons connected to active muscles, which can be mimicked in passive devices that rely only on elastic elements to reduce the cost of walking. For example, such passive ankle devices can reduce the metabolic cost of walking45 or running128,129,130. Furthermore, passive devices, which can be worn under clothes131, can assist the hip132 or ankle during walking.
Understanding the wearer’s response
Exoskeletons have begun to confer the wearer some of the long-hypothesized physiological benefits during locomotion. Devices for unimpaired users can reduce the effort of walking and jogging in lab-based and outdoor settings, and devices for gait rehabilitation have led to clinical improvements. Although the aim of devices for gait rehabilitation is to reduce the metabolic cost of walking, their immediate objective is to modify the wearer’s existing gait pattern. Accordingly, clinically relevant metrics and functional outcomes, such as walking speed and spatiotemporal symmetry, are used to evaluate device benefits. Therefore, understanding the wearer’s response to exoskeletons is crucial to enable the design of robust devices, individualized assistance and widespread user acceptance.
Reduction of metabolic cost
The first demonstration of an offboard ankle-assisting exoskeleton lowering the metabolic cost of walking compared to unassisted walking68 made clear the promise of joint-targeting exoskeletons. Joint-targeting devices can interface with the body through different approaches, and they can be designed with different actuation and control modes to assist a variety of joint motions, rather than just the ankle. The metabolic impact of these devices varies depending on whether the exoskeleton is autonomous or tethered to an offboard actuator, and on whether the effects of the exoskeleton are compared to walking without an exoskeleton (no exoskeleton) or to walking with an exoskeleton but without assistive torque (no assist).
Studying metabolic-cost reduction using offboard systems and comparisons to no assist allow for rapid iteration through experimental designs and through device and controller prototypes. An offboard ankle-assisting exosuit led to a magnitude of assistance delivered during walking that directly influenced metabolic reduction compared with no assist (22.83 ± 3.2%)82. Although the highest augmentation torques may achieve the highest metabolic reduction, well-optimized assistance profiles offer further metabolic benefits over fixed control strategies. Human-in-the-loop (HIL) optimization automatically individualizes control parameters to each participant in real time and has been shown to lead to metabolic reductions using offboard devices assisting the hip (17.4 ± 3.2% compared with no exoskeleton)85 and the ankle (24.3 ± 7.4% compared with no assist)78. Further study at the muscle level may provide more information on optimal profiles or device designs for maximizing metabolic reduction133.
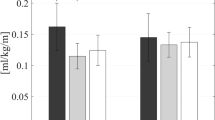
For unimpaired users, the ultimate goal of autonomous exoskeletons is to reduce the metabolic cost of walking relative to no exoskeleton; that is, these wearable devices should more than offset the additional weight (including that of power sources) carried by the user. Therefore, exoskeletons not only have to provide assistance at the correct time, but also have to be sufficiently light so that their metabolic benefit overcomes the metabolic burden of the added weight. A landmark study of an ankle-targeting autonomous system showed metabolic reduction during loaded walking (8 ± 3%) compared with no exoskeleton69. Other autonomous exoskeletons have also led to metabolic reductions relative to no exoskeleton134: a passive ankle device (7.2 ± 2.6%)45, a hip exoskeleton (GEMS, 17.4 ± 2.9%)135, and a soft exosuit for hip assistance during both walking (9.3 ± 2.2%)117 and running (4.0 ± 1.3%)117.
These results highlight the potential of exoskeletons as assistive complements to walking; however, their design, manufacturing and testing remain time-consuming and resource-consuming. In addition, predicting the efficiency of untested devices in metabolic-cost reduction remains challenging. The increase in metabolic cost owing to exoskeleton weight can be accounted for by the augmentation factor (AF), a metric aimed at predicting the metabolic benefit solely on the basis of its mass and power characteristics69. The AF is defined as
where p+ is the average positive augmentation power, pdis is the net augmentation-power dissipation (pdis = 0 when |p+| ≥ |p−| and pdis = p+ + p− when |p+| < |p−|; p− is the average negative-augmentation power), η is the muscle–tendon apparent efficiency15, mi are the added device masses on each segment (trunk, thigh, shank and foot) and βi are the device-location factors for each segment based on linear-regression equations136. When the AF was first introduced, only two datasets67,68, both assisting ankle plantarflexion, were available to estimate muscle–tendon apparent efficiency, η = 0.41, defined as
Using data published since 2014, focusing on studies demonstrating metabolic reductions relative to no assist, we calculated apparent joint efficiencies for ankle plantarflexion (η = 0.47)68,69,87,137,138,139 and hip extension (η = 0.49)117,140,141,142. Some studies looked at devices that assisted multiple joints14,143,144,145. However, some studies did not include all information necessary to compute equation (1), hence we made some assumptions about device parameters, such as weight distribution (Supplementary Table 1). Using these updated apparent joint efficiencies, the AF can predict the metabolic impact of exoskeletons (Fig. 3) for specific modes of assistance (Supplementary Table 2). Of note, a key assumption of the AF is that metabolic benefit from external assistance scales linearly with the average amount of positive power delivered by an exoskeleton. Some studies corroborate this assumption, showing, for example, that the condition with the highest augmentation power shows the highest metabolic-cost reduction82; or that two different assistance profiles with similar amounts of augmentation power lead to similar metabolic reductions84. By contrast, empirical experiments78,83,138 and simulations146,147 have indicated that there is a point at which too much augmentation power may not lead to additional metabolic-cost reductions. Moreover, studies using passive devices have shown the potential for low-positive-power strategies to reduce the metabolic cost of walking45.
Interestingly, data reported for elastic ankle exoskeletons with intermediate stiffness springs set in parallel with the human plantarflexors do not follow the general trend148. This study included actuation profiles that did not provide metabolic improvement; such actuation profiles were also included in another study for unpowered ankle exoskeletons45. Actuation profiles that do not decrease the metabolic cost of walking are not common in the recent literature, a gap that perhaps hides factors influencing metabolic benefit. Indeed, augmentation power is not the only factor influencing metabolic improvement. To change the sign of equation (1), the device has to apply substantially more negative augmentation power than positive augmentation power, or needs to be sufficiently heavy so that its distal inertia overrides any benefit of positive augmentation power. In addition, various other factors may influence the metabolic cost.
Device-location factors136, for example, do not account for the increased energy cost of moving distal mass at high walking speeds148. Moreover, the AF does not consider potentially substantial changes in kinematics owing to exoskeleton assistance82,148. Although incomplete, the AF remains a surprisingly powerful indicator of metabolic improvement of devices that apply net positive augmentation power. Studies describing actuation profiles that decrease and increase the metabolic cost of walking will further increase understanding of device designs that make walking easier.
In addition to the AF, other modelling techniques have been used to inform exoskeleton design with the aim of reducing metabolic cost. High-level models such as the individual-limbs method149 examine how each leg contributes mechanical power to the centre of mass150. Interestingly, high-level models have also been used to design a phase-oscillator controller that can reduce the metabolic cost of running compared with no exoskeleton151. Future approaches to predicting metabolic-cost reductions should also consider the cost difference in generating force with muscles crossing the hip, knee or ankle. Tools such as OpenSim152,153,154 can estimate individual muscle activations and kinetics, offering insights into how exoskeletons can modify muscle–tendon dynamics in ways that can either benefit155 or impede156 gait.
High-level models can provide information about key trade-offs in the early phases of device development, and more detailed musculoskeletal models can give insight into the biomechanical changes induced by wearable devices. To inform these models, close collaboration between device and simulation designers will be crucial. Experimentalists should provide key experimental data, such as applied device torques and powers, the distribution of added exoskeleton mass on the body, the resultant metabolic impact relative to both no exoskeleton and no assist, and raw kinetic, kinematic or muscle-level responses.
In-clinic validation
In contrast to devices designed for unimpaired users, improving walking performance in the clinic often does not focus on reducing metabolic cost. People with gait deficiencies develop atypical movement patterns that are thought to be necessary for stability, and devices applied to improve gait may therefore disrupt energetic efficiencies established through these compensations. Although metabolic cost remains important, clinical studies involving exoskeletons for gait assistance often focus on safety metrics to establish patient–device agreement, as well as on efficacy metrics based on walking function and quality. Here we discuss outcomes from exoskeleton evaluations in clinical populations ranging from case studies to randomized controlled trials (RCTs).
Weight-bearing exoskeletons have been studied in a range of conditions (from complete SCI to stroke) requiring full to partial assistance, whereas joint-targeting exoskeletons have mainly been used in conditions requiring partial assistance, such as stroke (Table 1). Only preliminary studies have been performed for assessing acceptability and feasibility of wearable exoskeletons in people with SCI thus far, owing to the complex nature of assisting gait in this condition. In clinical studies, exoskeletons have mainly been applied to increase training intensities to facilitate neuroplastic changes, as shown in animal models157; however, certain conditions, such as complete paralysis from SCI, benefit from exoskeletons as an alternative means of mobility.
Several commercial devices have been examined for their clinical potential, including Ekso158,159,160 and Indego161 for SCI, ReWalk for MS162, as well as HAL163 and ReStore122,164 for stroke. The results have shown acceptable feasibility and safety; however, occurrences of non-injurious falls158 and skin issues owing to device fit (such as redness and minor abrasion)122,161 were noted. Although assessments of device efficacy were limited in these preliminary studies, speed-based outcomes improved over the course of robotic gait training in the case of HAL for people with subacute stroke163, Ekso for people with SCI160 and ReWalk for people with MS162. In a multisession case study involving three participants with complete SCI, training with the Ekso resulted in linear increases in the number of steps and distances in 2 out of 3 participants, and a 2–3-fold increase in walking speed for all participants165. Notably, training with the ReStore resulted in a significant increase in unassisted maximum gait speeds of 0.07 m s−1 in 36 people with chronic stroke after a brief training of five sessions122, which is about a fourth of the dose of non-robotic training regimens, with a median dose of 20 sessions with pooled mean differences of 0.11 m s–1 (ref. 166). Training with the Indego device resulted in an average walking speed of 0.37 m s–1 in 32 individuals with SCI who were otherwise non-ambulatory without the device, after 26 visits161, which is about half the median dose of body-weight-supported treadmill-walking regimens (50 sessions), which did not increase walking speed167. In contrast to the aforementioned devices designed to provide assistance during walking, the Anklebot, which provides isolated training of the paretic ankle joint while seated, led to the transfer of gains from seated training to improvements in walking speed in 29 less-impaired individuals in the chronic phase of stroke168.
These findings highlight the potential of robot-assisted interventions in improving clinical outcomes. However, larger trials with control groups are needed to fully understand the generalizability of these results, the practicality of these technologies in the clinic and dose-response relationships over time. Importantly, exoskeletons must show clinically meaningful changes in walking performance in clinical trials. Specifically, RCTs seek to minimize biases in testing, thus increasing the likelihood of revealing robust effects of robotic training compared with conventional approaches169,170.
The effects of robotic training in people with stroke112,113,171,172,173,174,175,176 and other neurologic conditions, such as MS177,178 and TBI179, have been examined in RCTs. In people with chronic stroke, training with the SMA improved walking endurance, daily step count, corticomotor excitability of the paretic rectus femoris112, step length and spatial gait symmetry113, as compared with a control group who received non-robotic gait training only. Similarly, training with the Lokomat resulted in improvements in gait speed, balance and self-sufficiency175. Conversely, training with the Bionic Leg, a powered unilateral knee orthosis, failed to show benefits in gait speed in people with chronic stroke, and showed only modest functional benefits relative to gait training without robotics172.
Robotics have thus far only been applied in a subset of people with subacute stroke and substantial gait impairments. Training with the Lokomat173 and HAL174 led to a statistically significant increase in functional walking independence in people post-stroke, as well as to tendencies to increased walking speed following training with the treadmill-based Gait Assistance Robot171, as compared with gait training without robotics. Although less explored, early stroke rehabilitation during the acute phase or inpatient care may be one of the most relevant applications for exoskeletons. A large number of individuals with stroke are admitted to the hospital, and thus an ideal therapy design at this stage may be most advantageous, minimizing the development of compensations.
Training with the Lokomat also resulted in improvements in walking endurance and speed in people with MS, compared with conventional non-robotic training178. Training with the Gait Trainer resulted in improvements in balance; however, this effect was not significantly different compared to control training without robotics177. Favourable effects with the Lokomat were also observed in people with TBI, with an improvement in maximum walking speed and step length; however, walking endurance was only improved in non-robotic control training179.
Owing to the limited benefit of robot-assisted interventions over conventional gait training thus far24,25,29,30, treadmill-based exoskeletons are not currently clinically recommended for gait rehabilitation therapy of people with chronic stroke, incomplete SCI or TBI180. Nevertheless, the outcomes of RCTs can inform future clinical-trial designs to improve the quality and staging of the clinical studies with regards to appropriate outcome measures, dose-response effects and control groups for training-effect comparisons181,182. The device, training paradigms and patient-inclusion criteria need to be optimized to maximize the benefits of exoskeletons in gait rehabilitation183. A change in clinical practice is only justified if new interventions lead to a substantial benefit over existing practice180. Therefore, an RCT must be replicated across multiple testing sites to reduce bias and increase generalizability, which is regarded as the gold standard for therapeutic interventions181.
The pathway to multicentre RCTs with robotic devices should include four phases181. In phase 1, preliminary experiments should be conducted to confirm the safety of the device and its feasibility for clinical use. Clinically relevant outcome metrics, potential training paradigms and participant-inclusion criteria should also be determined at this phase. Once these phase-1 experiments confirm user safety and suggest improvements of clinical outcomes, then more detailed experiments, such as case studies with several participants, can be conducted in phase 2 to further inform training paradigms. Small case studies are more appropriate for substantiating preliminary phase-1 results in clinical settings, but do not require data from more participants than in phase 1. Case studies should include measurements of clinical and biomechanical metrics to enable preliminary explanations for high-level functional changes, such as gait speed. To provide initial insight into how the device compares to conventional therapy without robotics, case studies should involve a preliminary control group that receives similarly structured intervention without the device. In some cases, such as in the chronic phase of stroke, a crossover design can be applied; in this, the participants serve as their own control, and complete interventions with and without the device. Proper device validation in phases 1 and 2 aids in the formulation of the design of an RCT in phase 3 to ultimately enable evidence of intervention validity across multiple testing locations181 in phase 4. Therefore, if questions regarding intervention dosage, outcome metrics or participant inclusion remain unsolved on completion of phase 2, phases 1 and 2 should be repeated before progressing to phase 3. Similarly, if clinical outcomes are substantially improved but cannot be explained by quantitative biomechanical evaluations, phase 2 must be extended to better understand the human–robot interaction. Once functional outcomes have been shown to improve with the device with respect to the control, and once preliminary biological mechanisms have been identified, an RCT in phase 3 can be performed. An RCT must be sufficiently powered and contain a formal control group representative of usual care, to allow for comparisons of rehabilitation effects. If an RCT shows positive changes in some metrics but remains overall inconclusive, then progression to a multicentre RCT in phase 4 should be halted. Once clinically meaningful average improvements are obtained with the device (compared with the control group), a multicentre RCT can be conducted. Clinical effectiveness from such a study would motivate device implementation in clinical settings.
Only few exoskeleton systems have thus far been tested following this evidence-generation pathway. Although RCTs have shown promising clinical benefits related to speed and functional independence, these effects appear to be specific to the patient population and to the robotic devices (spanning a range of robotic systems, designs, controllers and interactions with diverse patient populations and impairment presentations184). It is important to identify which patient population would benefit the most from the robotic device185, rather than assessing a robot’s generalized benefit for all users184. Similarly, devices should be developed that can adapt to therapy, community settings and user needs to understand how exoskeletons can best support rehabilitation.
Therefore, clinical trials of neurorehabilitation involving robotics have to adopt strategic staging181,186, akin to stepwise and iterative processes employed in the development of drug-based therapies186, to minimize premature entry into multicentre RCTs. A comprehensive evaluation of effects of exoskeletons beyond functional outcomes is necessary to identify the underlying mechanisms that pertain to motor recovery versus compensations. However, clinical trials often lack the data required to perform quantitative biomechanical evaluations. Clinical outcomes, such as walking-speed improvements, may be similar in different interventions, but the underlying neural-control strategies may be distinct112,113. Robotic interventions can deliver high-intensity and high-repetition practice, which are known drivers of neuroplasticity and which should be considered in clinical trials involving robotics187. Understanding neuroplasticity mechanisms can drive neurotechnology design for rehabilitation188, considering that common neurorehabilitation practices value motor recovery over compensations. Spatiotemporal measurements from onboard sensors could shed light on the biomechanical mechanisms that lead to functional changes189,190; for example, by mapping neural signals obtained by electroencephalography (EEG) to gait kinematics191. In addition, temporal aspects of gait training should be considered. Clinical trials have thus far mainly focused on the rehabilitative potential of exoskeletons; however, the effective frequency and duration of training, and how long benefits may last, remain to be investigated185. Also, studies of assistive devices for continuous wear remain limited, but may allow for rehabilitation beyond the clinic and encourage community participation.
Component technology
Control
Accurate joint-angle estimation has proven invaluable in planning assistance. Most rigid systems use encoders on actuated joints to determine joint angles; however, devices without rigid joints cannot rely on encoders and thus must use other sensors (for example, inertial measurement units; IMUs192,193) to determine joint angles. IMUs are lightweight, inexpensive and easy to integrate into robotic systems, but also particularly sensitive to their physical alignment with biological joints (a challenge that rigid devices do not face). Furthermore, orientation measurements from IMUs suffer from inherent inaccuracies owing to bias (a constant offset compared to the true IMU orientation) and drift (a history-dependent change in orientation), which render measurements unusable within minutes if left uncorrected. Therefore, ensuring consistent alignment without first making precise measurements is difficult, hence IMU-based joint angles can be highly variable depending on placement. Importantly, asking wearers to take precise measurements each time they put on an exoskeleton hinders commercial applications, for which usability is crucial. Misalignments can be corrected by optimization algorithms that automatically estimate the location of the IMU relative to a biological joint, greatly improving joint-angle estimation accuracy194,195,196,197. Integrating these algorithms with wearable devices may enable the development of controllers that leverage joint-angle estimates for targeted assistance without requiring rigid joints. Such motion sensing could also be a valuable tool in the evaluation of a wearer’s kinematic performance outside of laboratory settings and could allow for the assessment of interventions at home or in the community. Furthermore, small IMUs worn during walking could inform an algorithm that determines the primary joint (or joints) at which a person experiences a gait deficit, along with the severity of that deficit. A clinician could then recommend a device appropriate for this specific deficit.
Exoskeletons are often controlled by applying actuation profiles as a function of the gait cycle (a standard time-normalized period that stretches from one heel strike to the next). Thus, parallels can be drawn between controllers, biological kinematics and kinetics. Heel-strike events can be assessed via the measurement of the foot switch, by placing an instrumented insole inside the shoe. Although simple in their design, foot switches often have issues related to sizing (the sensor must align well with both the heel and the ball of the foot) and durability (loading well exceeds body weight with every stride). Alternatively, IMUs can be used to detect heel strikes82,124. Detection of gait events is easiest with IMUs placed at the ankle, where the impact from a heel strike is most apparent. The cyclical nature of the gait cycle can also be leveraged by identifying measures in the phase plane that remain invariant across walking speeds198,199. Devices not targeting the ankle can be equipped with an adaptive oscillator99,100,105,106,107,108,200, which takes cyclic measurements of the hip angle to estimate the gait cycle. Instead of measuring the gait cycle, devices can also be designed to actuate in reaction to specific events as they occur119. Recent work has used machine-learning techniques to automatically detect the gait cycle using sensors at the hip201,202 and ankle203, adapted to different terrains and walking speeds.
The cyclical nature of walking enables the detection of repeated phases; however, gait initiation, termination, speed changes or turns can happen at any point during the gait cycle and are thus more challenging to detect. Therefore, exoskeletons often rely on sensor input to measure intent of movement and to predict the wearer’s motion before it occurs, to appropriately adjust assistance. However, detecting intent of movement is distinct from activity classification, in which heuristic methods or machine-learning techniques are used to identify activities beyond level-ground walking (such as walk-to-run transitions; or walking on terrains such as ramps or stairs204,205). Similar to detecting the gait phase, the intent of stepping can be detected using IMUs that measure joint angles and angular velocities206 and that estimate the centre-of-mass position64. Other motion-sensing modalities have also been integrated with lower-extremity exoskeletons to detect the intent of movement; for example, surface EMG can be applied to measure muscle activity and to administer proportional assistance at the ankle35. Myoelectric control can be further extended by integrating intricacies, such as adaptive gains137, and has been shown to be feasible in post-stroke gait207 across variable speeds208. However, surface EMG measures muscle activation rather than muscle motion, which may limit its ability to predict user intention. Alternatively, ultrasound imaging can be applied to sense differences in muscle dynamics for the detection of motor intent209. Such real-time estimation of muscle–tendon kinematic behaviour could be incorporated in exoskeleton-control schemes210. Although more common in upper-extremity exoskeletons, EEG for intent detection has also been explored in gait-related applications211. The NeuroRex was the first lower-extremity exoskeleton to integrate a brain–machine interface into its control loop, detecting a wearer’s motion intention to assist with sit-to-stand65 and walking66.
In functional electrical stimulation (FES), electrical impulses are delivered to a given muscle to generate involuntary contraction and to facilitate movement in paralyzed or weak limbs212,213,214. However, such external stimulation saturates muscle activation and can rapidly induce muscle fatigue, which increases the metabolic cost of walking compared with walking with an exoskeleton215. However, electrical stimulation can be combined with mechanical assistance. Although more common in upper-limb exoskeletons, electrical stimulation can also be integrated in lower-extremity exoskeletons to create hybrid systems for gait assistance216, particularly in people with paraplegia. The Vanderbilt Exoskeleton was the first hybrid system for paraplegic individuals. This hip-and-knee joint-coupled exoskeleton contains a push-button control to stimulate the quadriceps and to generate hip flexion and knee extension62. Kinesis, a knee-ankle-foot exoskeleton, similarly combines FES and mechanical assistance to balance the power contribution of the exoskeleton and muscle stimulation. Here, the closed-loop control of FES is based on EMG-estimated muscle fatigue217. Such hybrid systems are promising for promoting neuroplasticity because FES interfaces directly with the neuromuscular system and exoskeletons enable high training intensity. Hybrid FES–exoskeleton systems have mainly been explored for paraplegic applications thus far but may also benefit populations with more residual volitional contribution, such as people post-stroke.
Actuators
Actuator choice for exoskeletons is targeted towards limiting the distal mass and its costly metabolic impact218. Accordingly, actuators have been designed to minimize weight and to maximize power. For example, conventional motors can be applied to actuate rigid links and to establish sophisticated interfaces between exoskeletons and the body104,219,220. Bowden cables, which can transmit forces from a heavy actuator to lighter-weight distal components, have been applied in both autonomous117,118,145 and offboard devices77,79,82,85,115. Alternatively, McKibben-type pneumatic artificial muscles can distally apply sagittal-plane assistance34, with updated versions demonstrating metabolic benefit67,68,138,207. Other artificial-muscle technologies have also shown promise for reducing drop foot in people post-stroke221. Such soft inflatable actuators have been integrated into research devices for lateral ankle support222,223 and in commercial devices for recreational knee-injury prevention224. As a semi-active extension of a passive exoskeleton45, a proof-of-concept electroadhesive clutch has been designed to actively engage or disengage a spring when voltage is applied across a stack of thin electrode sheets225,226. These contrast with traditional mechanical clutches, which require a physical latch to engage or disengage a spring, requiring much more elaborate mechanisms. Electrostatic clutches could allow for the design of passive joint-targeting exoskeletons that adapt to changes in speed, movement and the environment.
Simulation of forward dynamics
High-fidelity simulations are an important tool for estimating the metabolic benefit of exoskeleton devices. Empirical techniques, such as HIL optimization, can optimize assistance in response to real-time measurements. In addition, the simulation of forward dynamics can be applied to analytically estimate the impact of exoskeletons, primarily in unimpaired users, but also in rehabilitation227. Forward-dynamics simulation can aid in the prototyping of new designs or modes of assistance, and in gaining insight into the biological mechanisms leading to empirical results. For example, exoskeletons have had limited success in lowering the metabolic cost of running; however, a simulation applying ideal torque actuators in seven different configurations to a running model228 could confirm experimentally observed phenomena, such as a decrease in muscle activation at unassisted joints115. A simulation of walking with heavy load suggested, somewhat surprisingly, that hip-abduction assistance could lower the metabolic cost of walking more than (the well-studied) ankle plantarflexion; however, this result remains to be experimentally confirmed153.
Machine learning for patient selection
Machine-learning techniques will allow researchers and clinicians to more efficiently identify people who could benefit from wearable devices. Machine learning can be applied to identify deficits in individuals and to predict the response to robotic interventions; this has been shown in upper-limb exoskeletons and could also be applied to lower-limb devices. For example, in upper-limb rehabilitation, an artificial neural network could predict changes in clinical scores throughout an 80-day exoskeleton-enabled training period in people post-stroke229. Similarly, baseline data from individuals with MS have been used to predict changes in clinical measures after 8 weeks of conventional rehabilitation230. A combination of movement presentations and demographic data has allowed for the subgrouping of patients to identify deficits in individuals231,232 and to find characteristic mechanisms that can explain differences between subgroups within a larger clinical population. These data-driven methods can also distinguish between categories of impairments233, and automated subgrouping methods can be applied to recruit representative participants for in-depth studies (for example, by using machine learning for patient selection234).
Outlook
Advances in exoskeleton technology at the system and component levels have contributed to the design of exoskeletons that are ready for clinical integration. Commercial devices are now available for clinical rehabilitation, and Food and Drug Administration (FDA)-approved exoskeletons can return walking ability to individuals who otherwise are unable to walk. Various research-grade exoskeleton devices have contributed to the understanding of the mechanisms by which users can leverage exoskeleton technology. However, clinical and commercial acceptance of exoskeletons remains limited; large-scale RCTs have shown inconclusive results, causing some clinical guidelines to recommend against the use of exoskeletons in gait-training applications for chronic stroke, SCI-i and TBI180. Moreover, the vision for recreational exoskeletons to extend walking capacity of unimpaired users remains to be fulfilled, and the driving factors for users to choose exoskeletons remain an important consideration.
Unimpaired users
Advances in sensing technologies and their integration with wearable devices will help reveal biological mechanisms to enable optimized control. For example, ultrasound imaging has been used with a soft exosuit to reduce the metabolic cost of walking by detecting instantaneous changes in muscle function and by modulating exosuit assistance accordingly210. Similar technologies could enable wearable devices to quickly adapt to individual wearers or unknown environments. Small body-worn sensors can measure muscle–tendon forces in real time in vivo235; if implemented in wearable robots, they could offer a metric of user effort and provide insight into how wearable devices can offload stress on soft tissue. Lightweight wearable sensors could be combined with exoskeletons to adapt body-worn devices to a range of activities, environments and wearers, by estimating kinematics during movement, as already demonstrated in the upper extremity with a soft sensing shirt236. Indeed, recent work with a joint-targeting autonomous ankle exoskeleton used wearable sensors and a data-driven model to predict the metabolic benefit of a particular control configuration. Using HIL optimization, exoskeleton assistance was adapted as the wearer walked outside the lab237. Real-time inverse-dynamics feedback has further been applied to show that healthy adults can accurately target a specified amount of ankle power238. Although limited to an instrumented treadmill and not yet suitable for autonomous exoskeletons, similar approaches may increase the understanding of how exoskeletons, which often augment the production of ankle power, interact with the body. Recent work has also used machine-learning techniques to estimate hip moments without requiring an instrumented treadmill239.
In addition to sensing modalities, new approaches to actuation and control can be integrated into exoskeletons. Muscle-inspired technologies, such as fibre-based actuators240 and other compliant actuators218, would enable soft actuators with an efficiency similar to biological actuators. Adding machine-learning techniques on top of more conventional control schemes such as adaptive oscillators may allow devices to quickly adapt to changes in walking condition241. The availability of more commercial devices will certainly contribute to a better understanding of the physiological impact of exoskeletons. Furthermore, accurate metabolic-cost estimates have to be implemented as real-time input to exoskeleton-control schemes to better prescribe assistance and to maximize individual metabolic-cost reductions. Ultimately, long-term investigations will be required to determine the relation of metabolic-cost reduction to a reduction in injury risk, an increase in wearer performance and an improvement in recreational capacity. These are especially important factors because it is unclear how sensitive individual wearers are to changes in metabolic cost242. Additional work may extend beyond metabolic cost to incorporate user preferences to rapidly adapt assistance to particular individuals and walking conditions243.
Patient populations
Multicentre RCTs are informative but are time-consuming and resource-consuming, which is not conducive to rapid prototype development244,245. Collaborations between clinicians and exoskeleton designers will enable targeted case studies with a small number of selected participants to inform biological mechanisms that warrant larger clinical trials and drive changes in functional outcomes, to enable the design of optimization algorithms for individualized assistance. Understanding disconnects between device function and clinical-trial goals, and quantifying challenges in clinical implementation will be instrumental in directing future studies. Importantly, patients should be included in the design of well-controlled case studies to understand how the devices can improve engagement in the clinical process, for example by following policies established for pharmaceutics246.
Rehabilitation studies with exoskeletons typically have the aim of demonstrating clinical efficacy (for example, an increase in walking speed) in a broad patient population, which is often divided into ‘responders’ and ‘non-responders’. A thorough understanding of the biomechanical and neurological mechanisms contributing to clinical outcomes in each subgroup would allow for the grouping of participants before recruitment. For example, machine-learning approaches may be applied to help clinicians prescribe a type of exoskeleton or a particular mode of assistance that will most probably improve walking for an individual user. Industry-wide standards for reproducibility that benchmark device comfort and utility will also give clinicians a clearer understanding of what clinical changes to expect247,248,249.
Online optimization methods that can identify optimal assistance profiles in healthy individuals78,85,250 may also apply to clinical populations. However, HIL techniques typically demand long experiment times, often requiring hours of continuous walking. For widespread application in the clinic, efficient automatic tuning algorithms will be needed that can tailor exoskeleton assistance to a single user.
Using kinematics data from an individual’s prior walking sessions with an exoskeleton may enable exoskeleton-assistance tuning without online optimization. This strategy has been employed for people post-stroke walking with an ankle-assisting wearable robot, where their pre-recorded walking data were used to selectively apply either positive or negative augmentation power251. However, to define optimal clinical outcomes, more than a single biomechanical objective is required. Therefore, appropriate clinical objectives that can be measured by devices need to be defined to be able to automatically modulate exoskeleton assistance to an individual patient’s goals. Warm-starting HIL algorithms with baseline data may enable devices to adapt to exoskeleton users as their gait changes across environments or with time. It may also be possible to predict the impact of robotic exoskeleton training before completing a full protocol, although such approaches have been validated only in the upper limb252. Ideally, the rehabilitation goals set by patients and clinicians will be achieved by automatic tuning of wearable-device settings. Individualizing assistance by tailoring physical designs to particular wearers through modular systems that can quickly switch from one target joint to another has proven encouraging in an early feasibility study253.
In addition to efficient patient selection, the temporal changes in the interaction between an individual and an exoskeleton need to be understood to be able to tailor individual training regimens. Neuromuscular studies provided insight into how individuals adapt to new environments, including the influence of wearable devices on ankle impedance254. This knowledge could be integrated in robotic rehabilitation. Studies of upper-limb rehabilitation have shown that it may be possible to distinguish between motor learning and adaptation to a device. Such data would allow for the interpretation of changes in response to training with an exoskeleton to address baseline impairment255. Locomotor-adaptation studies using the split-belt treadmill have revealed that people post-stroke showed improvements in gait symmetry after training with error augmentation in the short-term256, which can be extended to long-term retention by increasing training257 and translated to overground environments258. Furthermore, learning outcomes may be improved by appropriate intervention scheduling259 through intermittent exposure and by increasing the variability of training through the introduction of perturbations260,261. Challenging the user is also important, and an ‘optimal’ challenge may maximize retention261; for example, increasing propulsion demands using inclined split-belt treadmill training improves gait symmetry post-adaptation more than flat split-belt treadmill training in people post-stroke262; also, high-intensity robot-assisted gait training increases walking speed263. In addition to implicit learning pathways, rehabilitation schemes often also incorporate explicit learning through task-specific instructions or biofeedback, which are known to improve learning and rehabilitation outcomes264,265. Further investigating these variables in exoskeleton-enabled rehabilitation and long-term exoskeleton use will enable the design and development of training regimes that promote neuromotor recovery.
Interaction between clinicians and patients is often limited, with supervised therapy in the United States typically ending 6 months after a stroke, despite evidence of the benefits of long-term exercise266,267,268. The American Physical Therapy Association, an advocate of telehealth practices, recognizes the potential of expanded access to physical-therapy services269. Telehealth practices have been widely used in response to the COVID-19 pandemic and may continue to be applied. Determining how gait training is administered and monitored outside of the laboratory and the clinic becomes increasingly important270. Exoskeletons have the potential to be remote gait-training tools that supplement in-person therapy.
Devices designed for remote use could include real-time biometric monitoring of the wearer’s response; for example, skin-adhering flexible sensors can measure vital signs271. In addition to monitoring wearer safety, such sensor systems may be especially valuable in home or community environments for device control; that is, to modulate assistance and to encourage more user effort; or for user feedback, for example by providing a summary of exertion during walking. Integrating wearable sensors272 into wearable devices will enable more individualized and robust exoskeleton control, and improve remote-activity monitoring and performance reporting.
Ensuring user safety is a key exoskeleton-design consideration for unsupervised or remotely supervised community-based or home-based gait training. Unlike upper-extremity training, gait training inherently incurs a fall risk. Importantly, for gait training with a powered exoskeleton, wearer instability must be limited in the case of device failure. The unsupervised use of exoskeletons requires the design of streamlined devices that can be donned and operated by the wearer. Especially at the ankle, designers may wish to introduce additional active degrees of freedom to promote natural joint motion without sacrificing stability273. Improvements in adaptability and comfort will make future exoskeletons not only tools for gait training, but also all-day wear devices that increase the performance of everyday walking. To determine the fit and comfort during extended wear, metrics are needed that quantify device component fit274, and techniques are required to quantify the pressure exerted by exoskeleton attachments275. In addition, the human–machine interface needs to be closely monitored, in particular for users with reduced sensation in their impaired lower extremities.
Advances in exoskeleton technology will allow the integration of wearable devices in the daily lives of patients, clinicians and recreational users. Pioneering work in exoskeleton technology revealed how exoskeletons interact with (and modify) the basic physiological mechanisms of walking. This physiological understanding has made it possible for wearable robotic exoskeletons to reach clinical use. We believe that new component technologies will underpin the next decade of wearable robotics, and envision that exoskeletons will become an integral part of daily life.
References
Yagn, N. Apparatus for facilitating walking, running, and jumping. US patent 420,179 (1890).
Scholder, C. A. Movement-cure apparatus. US patent 675,678 (1901).
Büdingen, T. Movement-cure apparatus. US patent 964,898 (1910).
Cobb, G. L. Walking motion. US patent 2,010,482 (1935).
Pietro, F. Device for the automatic control of the articulation of the knee applicable to a prosthesis of the thigh. US patent 2,305,291 (1937).
Jansen, J. Phase I Report: DARPA Exoskeleton Program Technical Report January (Oak Ridge National Laboratory, 2004).
Kazerooni, H. & Steger, R. The Berkeley lower extremity exoskeleton. J. Dyn. Syst. Meas. Control 128, 14–25 (2006).
Zoss, A., Kazerooni, H. & Chu, A. Biomechanical design of the Berkeley lower extremity exoskeleton (BLEEX). IEEE/ASME Trans. Mechatron. 11, 128–138 (2006).
Guizzo, E. & Goldstein, H. The rise of the body bots. IEEE Spectr. 42, 50–56 (2005).
Walsh, C., Pasch, K. & Herr, H. An autonomous, underactuated exoskeleton for load-carrying augmentation. In IEEE/RSJ International Conference on Intelligent Robots and Systems 1410–1415 (IEEE, 2006).
Walsh, C. J. et al. Development of a lightweight, underactuated exoskeleton for load-carrying augmentation. In IEEE International Conference on Robotics and Automation 3485–3491 (IEEE, 2006).
Walsh, C. J. Biomimetic Design for an Under-actuated Leg Exoskeleton for Load-carrying Augmentation. PhD thesis, Massachusetts Institute of Technology (2006).
Valiente, A. Design of a Quasi-Passive Parallel Leg Exoskeleton to Augment Load Carrying for Walking. PhD thesis, Massachusetts Institute of Technology (2005).
Walsh, C. J., Endo, K. & Herr, H. A quasi-passive leg exoskeleton for load-carrying augmentation. Int. J. HR 04, 487–506 (2007).
Sawicki, G. S. & Ferris, D. P. Mechanics and energetics of level walking with powered ankle exoskeletons. J. Exp. Biol. 211, 1402–1413 (2008).
Gregorczyk, K. N. et al. Effects of a lower-body exoskeleton device on metabolic cost and gait biomechanics during load carriage. Ergonomics 53, 1263–1275 (2010).
Colombo, G., Joerg, M., Schreier, R. & Dietz, V. Treadmill training of paraplegic patients using a robotic orthosis. J. Rehabil. Res. Dev. 37, 693–700 (2000).
Hesse, S. & Uhlenbrock, D. A mechanized gait trainer for restoration of gait. J. Rehabil. Res. Dev. 37, 701–708 (2000).
Veneman, J. et al. Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 15, 379–386 (2007).
Banala, S. K., Agrawal, S. K. & Scholz, J. P. Active leg exoskeleton (ALEX) for gait rehabilitation of motor-impaired patients. In IEEE 10th International Conference on Rehabilitation Robotics 401–407 (IEEE, 2007).
Banala, S. K., Kim, S. H., Agrawal, S. K. & Scholz, J. P. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans Neural Syst. Rehabil. Eng. 17, 2–8 (2009).
Girone, M., Burdea, G., Bouzit, M., Popescu, V. & Deutsch, J. E. Orthopedic rehabilitation using the ‘rutgers ankle’ interface. Stud. Health Technol. Inform. 70, 89–95 (2000).
Kleim, J. A. & Jones, T. A. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 51, 225–239 (2008).
Hidler, J. et al. Multicenter randomized clinical trial evaluating the effectiveness of the lokomat in subacute stroke. Neurorehabil. Neural Repair 23, 5–13 (2009).
Hornby, T. G. et al. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 39, 1786–1792 (2008).
Husemann, B., Mu, F. & Krewer, C. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after. Stroke 38, 349–354 (2007).
Peurala, S. H., Tarkka, I. M., Pitkänen, K. & Sivenius, J. The effectiveness of body weight-supported gait training and floor walking in patients with chronic stroke. Arch. Phys. Med. Rehabil. 86, 1557–1564 (2005).
Peurala, S. H. et al. Effects of intensive therapy using gait trainer or floor walking exercises early after stroke. J. Rehabil. Med. 41, 166–173 (2009).
Werner, C., von Frankenberg, S., Treig, T., Konrad, M. & Hesse, S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke 33, 2895–2901 (2002).
Dobkin, B. H. & Duncan, P. W. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil. Neural Repair 26, 308–317 (2012).
Ferris, D. P., Czerniecki, J. M. & Hannaford, B. An ankle-foot orthosis powered by artificial pneumatic muscles. J. Appl. Biomech. 21, 189–197 (2005).
Ferris, D. P., Gordon, K. E., Sawicki, G. S. & Peethambaran, A. An improved powered ankle–foot orthosis using proportional myoelectric control. Gait Posture 23, 425–428 (2006).
Hollander, K. W., Ilg, R., Sugar, T. G. & Herring, D. An efficient robotic tendon for gait assistance. J. Biomech. Eng. 128, 788–791 (2006).
Gordon, K. E., Sawicki, G. S. & Ferris, D. P. Mechanical performance of artificial pneumatic muscles to power an ankle–foot orthosis. J. Biomech. 39, 1832–1841 (2006).
Gordon, K. E. & Ferris, D. P. Learning to walk with a robotic ankle exoskeleton. J. Biomech. 40, 2636–2644 (2007).
Beyl, P., Van Damme, M., Van Ham, R., Vanderborght, B. & Lefeber, D. Design and control of a lower limb exoskeleton for robot-assisted gait training. Appl. Bionics Biomech. 6, 229–243 (2009).
Jezernik, S., Jezernik, K. & Morari, M. Impedance Control Based Gait-Pattern Adaptation for a Robotic Rehabilitation Device. IFAC Proc. Volumes 35, 389–393 (2002).
Jezernik, S. & Morari, M. Controlling the human-robot interaction for robotic rehabilitation of locomotion. In Proc. 7th International Workshop on Advanced Motion Control 133–135 (IEEE, 2002).
Jezernik, S., Colombo, G. & Morari, M. Automatic gait-pattern adaptation algorithms for rehabilitation with a 4-DOF robotic orthosis. IEEE Trans. Robot. Autom. 20, 574–582 (2004).
Riener, R. et al. Patient-cooperative strategies for robot-aided treadmill training: first experimental results. IEEE Trans. Neural Syst. Rehabil. Eng. 13, 380–394 (2005).
Ekkelenkamp, R., Veltink, P., Stramigioli, S. & van der Kooij, H. Evaluation of a Virtual Model Control for the selective support of gait functions using an exoskeleton. In IEEE 10th International Conference on Rehabilitation Robotics 693–699 (IEEE, 2007).
Emken, J., Bobrow, J. & Reinkensmeyer, D. Robotic movement training as an optimization problem: designing a controller that assists only as needed. In IEEE 9th International Conference on Rehabilitation Robotics 307–312 (IEEE, 2005).
Emken, J. L., Harkema, S. J., Beres-Jones, J. A., Ferreira, C. K. & Reinkensmeyer, D. J. Feasibility of manual teach-and-replay and continuous impedance shaping for robotic locomotor training following spinal cord injury. IEEE Trans. Biomed. Eng. 55, 322–334 (2008).
Riener, R. The Cybathlon promotes the development of assistive technology for people with physical disabilities. J. Neuroeng. Rehabil. 13, 49 (2016).
Collins, S. H., Wiggin, M. B. & Sawicki, G. S. Reducing the energy cost of human walking using an unpowered exoskeleton. Nature 522, 212–215 (2015).
Dollar, A. M. & Herr, H. Lower extremity exoskeletons and active orthoses: challenges and state-of-the-art. IEEE Trans. Robot. 24, 144–158 (2008).
Young, A. J. & Ferris, D. P. State of the art and future directions for lower limb robotic exoskeletons. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 171–182 (2017).
Yan, T., Cempini, M., Oddo, C. M. & Vitiello, N. Review of assistive strategies in powered lower-limb orthoses and exoskeletons. Robot. Auton. Syst. 64, 120–136 (2015).
Louie, D. R. & Eng, J. J. Powered robotic exoskeletons in post-stroke rehabilitation of gait: a scoping review. J. Neuroeng. Rehabil. 13, 53 (2016).
Pennycott, A., Wyss, D., Vallery, H., Klamroth-Marganska, V. & Riener, R. Towards more effective robotic gait training for stroke rehabilitation: a review. J. Neuroeng. Rehabil. 9, 65 (2012).
Lajeunesse, V., Vincent, C., Routhier, F., Careau, E. & Michaud, F. Exoskeletons’ design and usefulness evidence according to a systematic review of lower limb exoskeletons used for functional mobility by people with spinal cord injury. Disabil. Rehabil. Assist. Technol. 11, 535–547 (2016).
Lim, D. et al. Development of a lower extremity exoskeleton robot with a quasi-anthropomorphic design approach for load carriage. In IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) 5345–5350 (IEEE, 2015).
Fontana, M., Vertechy, R., Marcheschi, S., Salsedo, F. & Bergamasco, M. The body extender: a full-body exoskeleton for the transport and handling of heavy loads. IEEE Robot. Autom. Mag. 21, 34–44 (2014).
De Looze, M. P., Bosch, T., Krause, F., Stadler, K. S. & O’Sullivan, L. W. Exoskeletons for industrial application and their potential effects on physical work load. Ergonomics 59, 671–681 (2016).
Kermavnar, T., de Vries, A. W., de Looze, M. P. & O’Sullivan, L. W. Effects of industrial back-support exoskeletons on body loading and user experience: an updated systematic review. Ergonomics 64, 685–711 (2020).
He, Y., Eguren, D., Luu, T. P. & Contreras-Vidal, J. L. Risk management and regulations for lower limb medical exoskeletons: a review. Med. Devices 10, 89–107 (2017).
Maeshima, S. et al. Efficacy of a hybrid assistive limb in post-stroke hemiplegic patients: a preliminary report. BMC Neurol. 11, 116 (2011).
Suzuki, K., Mito, G. & Kawamoto, H. Intention-based walking support for paraplegia patients with robot Suit HAL. Adv. Robot. 21, 1441–1469 (2007).
Esquenazi, A., Talaty, M., Packel, A. & Saulino, M. The Rewalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 91, 911–921 (2012).
Strausser, K. A. & Kazerooni, H. The development and testing of a human machine interface for a mobile medical exoskeleton. In IEEE/RSJ International Conference on Intelligent Robots and Systems 4911–4916 (IEEE, 2011).
Strausser, K. A., Swift, T. A., Zoss, A. B., Kazerooni, H. & Bennett, B. C. Mobile exoskeleton for spinal cord injury: development and testing. In Proc. ASME 2011 Dynamic Systems and Control Conference and Bath/ASME Symposium on Fluid Power and Motion Control Vol. 2, 419–425 (ASMEDC, 2011).
Farris, R. J., Quintero, H. A., Withrow, T. J. & Goldfarb, M. Design and simulation of a joint-coupled orthosis for regulating FES-aided gait. In IEEE International Conference on Robotics and Automation 1916–1922 (IEEE, 2009).
Kawamoto, H., Hayashi, T., Sakurai, T., Eguchi, K. & Sankai, Y. Development of single leg version of HAL for hemiplegia. in Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 5038–5043 (2009).
Wang, S. et al. Design and control of the MINDWALKER exoskeleton. IEEE Trans. Neural Syst. Rehabil. Eng. 23, 277–286 (2015).
Noda, T. et al. Brain-controlled exoskeleton robot for BMI rehabilitation. In 12th IEEE-RAS International Conference on Humanoid Robots (Humanoids 2012) 21–27 (IEEE, 2012).
Kilicarslan, A., Prasad, S., Grossman, R. G. & Contreras-Vidal, J. L. High accuracy decoding of user intentions using EEG to control a lower-body exoskeleton. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 5606–5609 (2013).
Sawicki, G. S. & Ferris, D. P. Powered ankle exoskeletons reveal the metabolic cost of plantar flexor mechanical work during walking with longer steps at constant step frequency. J. Exp. Biol. 212, 21–31 (2009).
Malcolm, P., Derave, W., Galle, S. & De Clercq, D. A simple exoskeleton that assists plantarflexion can reduce the metabolic cost of human walking. PLoS ONE 8, e56137 (2013).
Mooney, L. M., Rouse, E. J. & Herr, H. M. Autonomous exoskeleton reduces metabolic cost of human walking during load carriage. J. Neuroeng. Rehabil. 11, 80 (2014).
Mooney, L. M., Rouse, E. J. & Herr, H. M. Autonomous exoskeleton reduces metabolic cost of human walking. J. Neuroeng. Rehabil. 11, 151 (2014).
Roy, A. et al. Robot-aided neurorehabilitation: a novel robot for ankle rehabilitation. IEEE Trans. Robot. 25, 569–582 (2009).
Blaya, J. & Herr, H. Adaptive control of a variable-impedance ankle-foot orthosis to assist drop-foot gait. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 24–31 (2004).
Boehler, A. W., Hollander, K. W., Sugar, T. G. & Dosun S. Design, implementation and test results of a robust control method for a powered ankle foot orthosis (AFO). In IEEE International Conference on Robotics and Automation 2025–2030 (IEEE, 2008).
Hitt, J. et al. Dynamically controlled ankle-foot orthosis (DCO) with regenerative kinetics: incrementally attaining user portability. In IEEE International Conference on Robotics and Automation 1541–1546 (IEEE, 2007).
Roy, A., Krebs, H. I., Barton, J. E., Macko, R. F. & Forrester, L. W. Anklebot-assisted locomotor training after stroke: a novel deficit-adjusted control approach. In IEEE International Conference on Robotics and Automation 2175–2182 (IEEE, 2013).
Witte, K. A., Zhang, J., Jackson, R. W. & Collins, S. H. Design of two lightweight, high-bandwidth torque-controlled ankle exoskeletons. In IEEE International Conference on Robotics and Automation 1223–1228 (IEEE, 2015).
Caputo, J. M. & Collins, S. H. A universal ankle–foot prosthesis emulator for human locomotion experiments. J. Biomech. Eng. 136, 035002 (2014).
Zhang, J. et al. Human-in-the-loop optimization of exoskeleton assistance during walking. Science 356, 1280–1284 (2017).
Ding, Y. et al. Multi-joint actuation platform for lower extremity soft exosuits. In IEEE International Conference on Robotics and Automation 1327–1334 (IEEE, 2014).
Bae, J. et al. A soft exosuit for patients with stroke: feasibility study with a mobile off-board actuation unit. In IEEE International Conference on Rehabilitation Robotics 131–138 (IEEE, 2015).
Bryan, G. M., Franks, P. W., Klein, S. C., Peuchen, R. J. & Collins, S. H. A hip–knee–ankle exoskeleton emulator for studying gait assistance. Int. J. Robot. Res. 40, 722–746 (2021).
Quinlivan, B. T. et al. Assistance magnitude versus metabolic cost reductions for a tethered multiarticular soft exosuit. Sci. Robot. 2, eaah4416 (2017).
Jackson, R. W. & Collins, S. H. An experimental comparison of the relative benefits of work and torque assistance in ankle exoskeletons. J. Appl. Physiol. 119, 541–557 (2015).
Grimmer, M. et al. Comparison of the human-exosuit interaction using ankle moment and ankle positive power inspired walking assistance. J. Biomech. 83, 76–84 (2019).
Ding, Y., Kim, M., Kuindersma, S. & Walsh, C. J. Human-in-the-loop optimization of hip assistance with a soft exosuit during walking. Sci. Robot. 3, eaar5438 (2018).
Hidayah, R. et al. Gait adaptation using a cable-driven active leg exoskeleton (C-ALEX) with post-stroke participants. IEEE Trans. Neural Syst. Rehabil. Eng. 28, 1984–1993 (2020).
Mooney, L. M. & Herr, H. M. Biomechanical walking mechanisms underlying the metabolic reduction caused by an autonomous exoskeleton. J. Neuroeng. Rehabil. 13, 4 (2016).
Giovacchini, F. et al. A light-weight active orthosis for hip movement assistance. Robot. Auton. Syst. 73, 123–134 (2015).
Kang, I., Hsu, H. & Young, A. J. Design and validation of a torque controllable hip exoskeleton for walking assistance. In ASME 2018 Dynamic Systems and Control Conference https://doi.org/10.1115/DSCC2018-9198 (2018).
Kang, I., Kunapuli, P., Hsu, H. & Young, A. J. Electromyography (EMG) signal contributions in speed and slope estimation using robotic exoskeletons. In IEEE International Conference on Rehabilitation Robotics 548–553 (IEEE, 2019).
Martini, E. et al. Gait training using a robotic hip exoskeleton improves metabolic gait efficiency in the elderly. Sci. Rep. 9, 7157 (2019).
Lerner, Z. F., Damiano, D. L., Park, H.-S., Gravunder, A. J. & Bulea, T. C. A robotic exoskeleton for treatment of crouch gait in children with cerebral palsy: design and initial application. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 650–659 (2017).
Lerner, Z. F., Damiano, D. L. & Bulea, T. C. A lower-extremity exoskeleton improves knee extension in children with crouch gait from cerebral palsy. Sci. Transl. Med. 9, eaam9145 (2017).
Lv, G., Zhu, H. & Gregg, R. D. On the design and control of highly backdrivable lower-limb exoskeletons: a discussion of past and ongoing work. IEEE Control Syst. 38, 88–113 (2018).
Sanz-Morere, C. B. et al. A bioinspired control strategy for the CYBERLEGs knee-ankle-foot orthosis: feasibility study with lower-limb amputees. In 7th IEEE International Conference on Biomedical Robotics and Biomechatronics 503–508 (IEEE, 2018).
Shimada, H. et al. The use of positron emission tomography and [18F]fluorodeoxyglucose for functional imaging of muscular activity during exercise with a stride assistance system. IEEE Trans. Neural Syst. Rehabil. Eng. 15, 442–448 (2007).
Shimada, H. et al. Effects of an automated stride assistance system on walking parameters and muscular glucose metabolism in elderly adults. Br. J. Sports Med. 42, 622–629 (2008).
Shimada, H. et al. Effects of a robotic walking exercise on walking performance in community-dwelling elderly adults. Geriatr. Gerontol. Int. 9, 372–381 (2009).
Seo, K., Hyung, S., Choi, B. K., Lee, Y. & Shim, Y. A new adaptive frequency oscillator for gait assistance. In IEEE International Conference on Robotics and Automation 5565–5571 (IEEE, 2015).
Seo, K., Lee, J., Lee, Y., Ha, T. & Shim, Y. Fully autonomous hip exoskeleton saves metabolic cost of walking. In IEEE International Conference on Robotics and Automation 4628–4635 (IEEE, 2016).
Nagarajan, U., Aguirre-Ollinger, G. & Goswami, A. Integral admittance shaping for exoskeleton control. In IEEE International Conference on Robotics and Automation 5641–5648 (IEEE, 2015).
Nagarajan, U. & Goswami, A. Improved mobility with a neutral, motion-amplifying controller for an experimental exoskeleton. SAE Int. J. Passeng. Cars Mech. Syst. 8, 606–6131 (2015).
Aguirre-Ollinger, G., Nagarajan, U. & Goswami, A. An admittance shaping controller for exoskeleton assistance of the lower extremities. Auton. Robots 40, 701–728 (2016).
Lee, Y. et al. A flexible exoskeleton for hip assistance. In IEEE/RSJ International Conference on Intelligent Robots and Systems 1058–1063 (IEEE, 2017).
Seo, K., Lee, J. & Park, Y. J. Autonomous hip exoskeleton saves metabolic cost of walking uphill. In International Conference on Rehabilitation Robotics 246–251 (IEEE, 2017).
Lee, J. et al. Effects of assistance timing on metabolic cost, assistance power, and gait parameters for a hip-type exoskeleton. In International Conference on Rehabilitation Robotics 498–504 (IEEE, 2017).
Lee, H.-J. et al. A wearable hip assist robot can improve gait function and cardiopulmonary metabolic efficiency in elderly adults. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 1549–1557 (2017).
Lee, S.-H. et al. Gait performance and foot pressure distribution during wearable robot-assisted gait in elderly adults. J. Neuroeng. Rehabil. 14, 123 (2017).
Orekhov, G., Fang, Y., Cuddeback, C. F. & Lerner, Z. F. Usability and performance validation of an ultra-lightweight and versatile untethered robotic ankle exoskeleton. J. Neuroeng. Rehabil. 18, 163 (2021).
Conner, B., Orekhov, G. & Lerner, Z. Ankle exoskeleton assistance increases six-minute walk test performance in cerebral palsy. IEEE Open J. Eng. Med. Biol. 2, 320–323 (2021).
Pour Aji Bishe, S. S., Liebelt, L., Fang, Y. & Lerner, Z. F. A low-profile hip exoskeleton for pathological gait assistance: design and pilot testing. In IEEE International Conference on Robotics and Automation 5461–5466 (2022).
Jayaraman, A. et al. Stride management assist exoskeleton vs functional gait training in stroke. Neurology 92, e263–e273 (2019).
Buesing, C. et al. Effects of a wearable exoskeleton stride management assist system (SMA®) on spatiotemporal gait characteristics in individuals after stroke: a randomized controlled trial. J. Neuroeng. Rehabil. 12, 69 (2015).
Wehner, M. et al. A lightweight soft exosuit for gait assistance. In IEEE International Conference on Robotics and Automation 3362–3369 (IEEE, 2013).
Lee, G. et al. Reducing the metabolic cost of running with a tethered soft exosuit. Sci. Robot. 2, eaan6708 (2017).
Lee, S. et al. Autonomous multi-joint soft exosuit with augmentation-power-based control parameter tuning reduces energy cost of loaded walking. J. Neuroeng. Rehabil. 15, 66 (2018).
Kim, J. et al. Reducing the metabolic rate of walking and running with a versatile, portable exosuit. Science 365, 668–672 (2019).
Kim, J. et al. Autonomous and portable soft exosuit for hip extension assistance with online walking and running detection algorithm. In IEEE International Conference on Robotics and Automation 5473–5480 (IEEE, 2018).
Bae, J. et al. A lightweight and efficient portable soft exosuit for paretic ankle assistance in walking after stroke. In IEEE International Conference on Robotics and Automation 2820–2827 (IEEE, 2018).
Awad, L. N. et al. A soft robotic exosuit improves walking in patients after stroke. Sci. Transl. Med. 9, eaai9084 (2017).
Awad, L. N. et al. Reducing circumduction and hip hiking during hemiparetic walking through targeted assistance of the paretic limb using a soft robotic exosuit. Am. J. Phys. Med. Rehabil. 96, S157–S164 (2017).
Awad, L. N., Esquenazi, A., Francisco, G. E., Nolan, K. J. & Jayaraman, A. The ReWalk ReStoreTM soft robotic exosuit: a multi-site clinical trial of the safety, reliability, and feasibility of exosuit-augmented post-stroke gait rehabilitation. J. Neuroeng. Rehabil. 17, 80 (2020).
Schmidt, K. et al. The Myosuit: bi-articular anti-gravity exosuit that reduces hip extensor activity in sitting transfers. Front. Neurorobot. 11, 57 (2017).
Haufe, F. L. et al. User-driven walking assistance: first experimental results using the MyoSuit. IEEE Int. Conf. Rehabil. Robot. 2019, 944–949 (2019).
Kwon, J. et al. A soft wearable robotic ankle-foot-orthosis for post-stroke patients. IEEE Robot. Autom. Lett. 4, 2547–2552 (2019).
Murakami, K., John, S. W., Komatsu, M. & Adachi, S. External control of walking direction, using cross-wire mobile assist suit. In IEEE/RSJ International Conference on Intelligent Robots and Systems 1046–1051 (IEEE, 2017).
Di Natali, C. et al. Design and evaluation of a soft assistive lower limb exoskeleton. Robotica 37, 2014–2034 (2019).
Nasiri, R., Ahmadi, A. & Ahmadabadi, M. N. Reducing the energy cost of human running using an unpowered exoskeleton. IEEE Trans. Neural Syst. Rehabil. Eng. 26, 2026–2032 (2018).
Cherry, M. S., Kota, S. & Ferris, D. P. An Elastic Exoskeleton for Assisting Human Running. In Proc. ASME 2009 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference Vol. 7, 727–738 (ASME, 2009).
Cherry, M. S., Kota, S., Young, A. & Ferris, D. P. Running with an elastic lower limb exoskeleton. J. Appl. Biomech. 32, 269–277 (2016).
Yandell, M. B., Tacca, J. R. & Zelik, K. E. Design of a low profile, unpowered ankle exoskeleton that fits under clothes: overcoming practical barriers to widespread societal adoption. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 712–723 (2019).
Panizzolo, F. A., Bolgiani, C., Di Liddo, L., Annese, E. & Marcolin, G. Reducing the energy cost of walking in older adults using a passive hip flexion device. J. Neuroeng. Rehabil. 16, 117 (2019).
Nuckols, R. W., Dick, T. J. M., Beck, O. N. & Sawicki, G. S. Ultrasound imaging links soleus muscle neuromechanics and energetics during human walking with elastic ankle exoskeletons. Sci. Rep. 10, 3604 (2020).
Sawicki, G. S., Beck, O. N., Kang, I. & Young, A. J. The exoskeleton expansion: improving walking and running economy. J. Neuroeng. Rehabil. 17, 25 (2020).
Lim, B. et al. Delayed output feedback control for gait assistance and resistance using a robotic exoskeleton. IEEE Robot. Autom. Lett. 4, 3521–3528 (2019).
Browning, R. C., Modica, J. R., Kram, R. & Goswami, A. The effects of adding mass to the legs on the energetics and biomechanics of walking. Med. Sci. Sports Exerc. 39, 515–525 (2007).
Koller, J. R., Jacobs, D. A., Ferris, D. P. & Remy, C. D. Learning to walk with an adaptive gain proportional myoelectric controller for a robotic ankle exoskeleton. J. Neuroeng. Rehabil. 12, 97 (2015).
Galle, S., Malcolm, P., Collins, S. H. & De Clercq, D. Reducing the metabolic cost of walking with an ankle exoskeleton: interaction between actuation timing and power. J. Neuroeng. Rehabil. 14, 35 (2017).
Khazoom, C. et al. Design and control of a multifunctional ankle exoskeleton powered by magnetorheological actuators to assist walking, jumping, and landing. IEEE Robot. Autom. Lett. 4, 3083–3090 (2019).
Ding, Y. et al. Effect of timing of hip extension assistance during loaded walking with a soft exosuit. J. Neuroeng. Rehabil. 13, 87 (2016).
Ding, Y. et al. Biomechanical and physiological evaluation of multi-joint assistance with soft exosuits. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 119–130 (2017).
Panizzolo, F. A. et al. Metabolic cost adaptations during training with a soft exosuit assisting the hip joint. Sci. Rep. 9, 9779 (2019).
Donelan, J. M. et al. Biomechanical energy harvesting: generating electricity during walking with minimal user effort. Science 319, 807–810 (2008).
van Dijk, W., van der Kooij, H. & Hekman, E. A passive exoskeleton with artificial tendons: design and experimental evaluation. IEEE Int. Conf. Rehabil. Robot. 2011, 5975470 (2011).
Panizzolo, F. A. et al. A biologically-inspired multi-joint soft exosuit that can reduce the energy cost of loaded walking. J. Neuroeng. Rehabil. 13, 43 (2016).
Zelik, K. E., Huang, T.-W. P., Adamczyk, P. G. & Kuo, A. D. The role of series ankle elasticity in bipedal walking. J. Theor. Biol. 346, 75–85 (2014).
Robertson, B. D., Farris, D. J. & Sawicki, G. S. More is not always better: modeling the effects of elastic exoskeleton compliance on underlying ankle muscle–tendon dynamics. Bioinsp. Biomim. 9, 046018 (2014).
Nuckols, R. W. & Sawicki, G. S. Impact of elastic ankle exoskeleton stiffness on neuromechanics and energetics of human walking across multiple speeds. J. Neuroeng. Rehabil. 17, 75 (2020).
Donelan, J. M., Kram, R. & Kuo, A. D. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J. Exp. Biol. 205, 3717–3727 (2002).
Wong, J. D. & Donelan, J. M. in Humanoid Robotics: A Reference (eds Goswami, A. & Vadakkepat, P.) 1–28 (Springer, 2017).
Sugar, T. G. et al. Limit cycles to enhance human performance based on phase oscillators. J. Mech. Robot. 7, 011001 (2015).
Uchida, T. K., Hicks, J. L., Dembia, C. L. & Delp, S. L. Stretching your energetic budget: how tendon compliance affects the metabolic cost of running. PLoS ONE 11, e0150378 (2016).
Dembia, C. L., Silder, A., Uchida, T. K., Hicks, J. L. & Delp, S. L. Simulating ideal assistive devices to reduce the metabolic cost of walking with heavy loads. PLoS ONE 12, e0180320 (2017).
Slade, P., Troutman, R., Kochenderfer, M. J., Collins, S. H. & Delp, S. L. Rapid energy expenditure estimation for ankle assisted and inclined loaded walking. J. Neuroeng. Rehabil. 16, 67 (2019).
Durandau, G., Rampeltshammer, W. F., van der Kooij, H. & Sartori, M. Neuromechanical model-based adaptive control of bilateral ankle exoskeletons: biological joint torque and electromyogram reduction across walking conditions. IEEE Trans. Robot. 38, 1380–1394 (2022).
Farris, D. J., Hicks, J. L., Delp, S. L. & Sawicki, G. S. Musculoskeletal modelling deconstructs the paradoxical effects of elastic ankle exoskeletons on plantar-flexor mechanics and energetics during hopping. J. Exp. Biol. 217, 4018–4028 (2014).
See, P. A. & de Leon, R. D. Robotic loading during treadmill training enhances locomotor recovery in rats spinally transected as neonates. J. Neurophysiol. 110, 760–767 (2013).
Kolakowsky-Hayner, S. A., Crew, J., Moran, S. & Shah, A. Safety and feasibility of using the EksoTM bionic exoskeleton to aid ambulation after spinal cord injury. J. Spine S4, 003 (2013).
Kozlowski, A., Bryce, T. & Dijkers, M. Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top. Spinal Cord Inj. Rehabil. 21, 110–121 (2015).
Sale, P. et al. Effects on mobility training and de-adaptations in subjects with spinal cord injury due to a wearable robot: a preliminary report. BMC Neurol. 16, 12 (2016).
Tefertiller, C. et al. Initial outcomes from a multicenter study utilizing the indego powered exoskeleton in spinal cord injury. Top. Spinal Cord Inj. Rehabil. 24, 78–85 (2018).
Kozlowski, A. J., Fabian, M., Lad, D. & Delgado, A. D. Feasibility and safety of a powered exoskeleton for assisted walking for persons with multiple sclerosis: a single-group preliminary study. Arch. Phys. Med. Rehabil. 98, 1300–1307 (2017).
Nilsson, A. et al. Gait training early after stroke with a new exoskeleton – the hybrid assistive limb: a study of safety and feasibility. J. Neuroeng. Rehabil. 11, 92 (2014).
Shin, S. Y. et al. Soft robotic exosuit augmented high intensity gait training on stroke survivors: a pilot study. J. Neuroeng. Rehabil. 19, 51 (2022).
Kressler, J. et al. Understanding therapeutic benefits of overground bionic ambulation: exploratory case series in persons with chronic, complete spinal cord injury. Arch. Phys. Med. Rehabil. 95, 1878–1887 (2014).
Saunders, D. H. et al. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2020, CD003316 (2020).
Mehrholz, J., Kugler, J. & Pohl, M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst. Rev. 2012, CD006676 (2012).
Chang, J. L. et al. Intensive seated robotic training of the ankle in patients with chronic stroke differentially improves gait. NeuroRehabilitation 41, 61–68 (2017).
Darrah, J., Hickman, R., O’Donnell, M., Vogtle, L. & Wiart, L. AACPDM Methodology to Develop Systematic Reviews of Treatment Interventions (Revision 1.2) (AACPDM, 2008); https://www.aacpdm.org/UserFiles/file/systematic-review-methodology.pdf
Howick, J. et al. The 2011 Oxford Levels of Evidence (Centre for Evidence-Based Medicine, 2011); http://www.cebm.net/index.aspx?o=5653
Ochi, M., Wada, F., Saeki, S. & Hachisuka, K. Gait training in subacute non-ambulatory stroke patients using a full weight-bearing gait-assistance robot: a prospective, randomized, open, blinded-endpoint trial. J. Neurol. Sci. 353, 130–136 (2015).
Stein, J., Bishop, L., Stein, D. J. & Wong, C. K. Gait training with a robotic leg brace after stroke. Am. J. Phys. Med. Rehabil. 93, 987–994 (2014).
Taveggia, G., Borboni, A., Mule, C., Villafañe, J. H. & Negrini, S. Conflicting results of robot-assisted versus usual gait training during postacute rehabilitation of stroke patients. Int. J. Rehabil. Res. 39, 29–35 (2016).
Watanabe, H., Tanaka, N., Inuta, T., Saitou, H. & Yanagi, H. Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: a randomized controlled pilot study. Arch. Phys. Med. Rehabil. 95, 2006–2012 (2014).
Bang, D.-H. & Shin, W.-S. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: a randomized controlled pilot trial. NeuroRehabilitation 38, 343–349 (2016).
Yeung, L.-f et al. Randomized controlled trial of robot-assisted gait training with dorsiflexion assistance on chronic stroke patients wearing ankle-foot-orthosis. J. Neuroeng. Rehabil. 15, 51 (2018).
Gandolfi, M. et al. Robot-assisted vs. sensory integration training in treating gait and balance dysfunctions in patients with multiple sclerosis: a randomized controlled trial. Front. Hum. Neurosci. 8, 318 (2014).
Straudi, S. et al. Does robot-assisted gait training ameliorate gait abnormalities in multiple sclerosis? A pilot randomized-control trial. NeuroRehabilitation 33, 555–563 (2013).
Esquenazi, A., Lee, S., Packel, A. T. & Braitman, L. A randomized comparative study of manually assisted versus robotic-assisted body weight supported treadmill training in persons with a traumatic brain injury. PM R 5, 280–290 (2013).
Hornby, T. G. et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J. Neurol. Phys. Ther. 44, 49–100 (2020).
Dobkin, B. H. Progressive staging of pilot studies to improve phase iii trials for motor interventions. Neurorehabil. Neural Repair 23, 197–206 (2009).
Lo, A. C. Clinical designs of recent robot rehabilitation trials. Am. J. Phys. Med. Rehabil. 91, S204–S216 (2012).
Winstein, C. J. et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 47, e98–e169 (2016).
Morone, G. et al. Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr. Dis. Treat. 13, 1303–1311 (2017).
Mehrholz, J. et al. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 5, CD006185 (2017).
Field-Fote, E. E. Lessons from COVID-19 on the stepwise development of interventions. J. Neurol. Phys. Ther. 44, 177–178 (2020).
Bowden, M. G. et al. Advancing measurement of locomotor rehabilitation outcomes to optimize interventions and differentiate between recovery versus compensation. J. Neurol. Phys. Ther. 36, 38–44 (2012).
Micera, S., Caleo, M., Chisari, C., Hummel, F. C. & Pedrocchi, A. Advanced neurotechnologies for the restoration of motor function. Neuron 105, 604–620 (2020).
Dobkin, B. H. & Martinez, C. Wearable sensors to monitor, enable feedback, and measure outcomes of activity and practice. Curr. Neurol. Neurosci. Rep. 18, 87 (2018).
Channa, A., Popescu, N. & Ciobanu, V. Wearable solutions for patients with Parkinson’s disease and neurocognitive disorder: a systematic review. Sensors 20, 2713 (2020).
Contreras-Vidal, J. L. et al. Neural decoding of robot-assisted gait during rehabilitation after stroke. Am. J. Phys. Med. Rehabil. 97, 541–550 (2018).
Malcolm, P. et al. Varying negative work assistance at the ankle with a soft exosuit during loaded walking. J. Neuroeng. Rehabil. 14, 62 (2017).
Lee, S. et al. Controlling negative and positive power at the ankle with a soft exosuit. In IEEE International Conference on Robotics and Automation 3509–3515 (IEEE, 2016).
Seel, T., Schauer, T. & Raisch, J. Joint axis and position estimation from inertial measurement data by exploiting kinematic constraints. In IEEE International Conference on Control Applications 45–49 (2012).
Seel, T., Raisch, J. & Schauer, T. IMU-based joint angle measurement for gait analysis. Sensors 14, 6891–6909 (2014).
Laidig, D., Schauer, T. & Seel, T. Exploiting kinematic constraints to compensate magnetic disturbances when calculating joint angles of approximate hinge joints from orientation estimates of inertial sensors. In International Conference on Rehabilitation Robotics 971–976 (IEEE, 2017).
Picerno, P. 25 years of lower limb joint kinematics by using inertial and magnetic sensors: a review of methodological approaches. Gait Posture 51, 239–246 (2017).
Gregg, R. D., Rouse, E. J., Hargrove, L. J. & Sensinger, J. W. Evidence for a time-invariant phase variable in human ankle control. PLoS ONE 9, e89163 (2014).
Quintero, D., Villarreal, D. J. & Gregg, R. D. Preliminary experiments with a unified controller for a powered knee-ankle prosthetic leg across walking speeds. In IEEE/RSJ International Conference on Intelligent Robots and Systems 5427–5433 (IEEE, 2016).
Lee, Y. et al. A flexible exoskeleton for hip assistance. In IEEE/RSJ International Conference on Intelligent Robots and Systems 1058–1063 (IEEE, 2017).
Kang, I., Kunapuli, P. & Young, A. J. Real-time neural network-based gait phase estimation using a robotic hip exoskeleton. IEEE Trans. Med. Robot. Bionics 2, 28–37 (2020).
Kang, I. et al. Real-time gait phase estimation for robotic hip exoskeleton control during multimodal locomotion. IEEE Robot. Autom. Lett. 6, 3491–3497 (2021).
Shepherd, M. K., Molinaro, D. D., Sawicki, G. S. & Young, A. J. Deep learning enables exoboot control to augment variable-speed walking. IEEE Robot. Autom. Lett. 7, 3571–3577 (2022).
Al-dabbagh, A. H. & Ronsse, R. A review of terrain detection systems for applications in locomotion assistance. Robot. Auton. Syst. 133, 103628 (2020).
Kang, I., Molinaro, D. D., Choi, G., Camargo, J. & Young, A. J. Subject-independent continuous locomotion mode classification for robotic hip exoskeleton applications. IEEE Trans. Biomed. Eng. 69, 3234–3242 (2022).
Novak, D. et al. Automated detection of gait initiation and termination using wearable sensors. Med. Eng. Phys. 35, 1713–1720 (2013).
Takahashi, K. Z., Lewek, M. D. & Sawicki, G. S. A neuromechanics-based powered ankle exoskeleton to assist walking post-stroke: a feasibility study. J. Neuroeng. Rehabil. 12, 23 (2015).
McCain, E. M. et al. Mechanics and energetics of post-stroke walking aided by a powered ankle exoskeleton with speed-adaptive myoelectric control. J. Neuroeng. Rehabil. 16, 57 (2019).
Hariharan, H. et al. in Medical Imaging 2016: Ultrasonic Imaging and Tomography (eds Duric, N. & Heyde, B.) 97901Q (SPIE, 2016).
Nuckols, R. W. et al. Automated detection of soleus concentric contraction in variable gait conditions for improved exosuit control. In IEEE International Conference on Robotics and Automation 4855–4862 (IEEE, 2020).
Tariq, M., Trivailo, P. M. & Simic, M. EEG-based BCI control schemes for lower-limb assistive-robots. Front. Hum. Neurosci. 12, 312 (2018).
Popovic, M., Keller, T., Papas, I., Dietz, V. & Morari, M. Surface-stimulation technology for grasping and walking neuroprostheses. IEEE Eng. Med. Biol. Mag. 20, 82–93 (2001).
Thrasher, T. & Popovic, M. Functional electrical stimulation of walking: function, exercise and rehabilitation. Ann. Readapt. Med. Phys. 51, 452–460 (2008).
Sartori, M. & Sawicki, G. Closing the loop between wearable technology and human biology: a new paradigm for steering neuromuscular form and function. Prog. Biomed. Eng. 3, 023001 (2021).
Khan, A. S. et al. Retraining walking over ground in a powered exoskeleton after spinal cord injury: a prospective cohort study to examine functional gains and neuroplasticity. J. Neuroeng. Rehabil. 16, 145 (2019).
del-Ama, A. J. et al. Review of hybrid exoskeletons to restore gait following spinal cord injury. J. Rehabil. Res. Dev. 49, 497 (2012).
del-Ama, A. J., Gil-Agudo, A. ́, Pons, J. L. & Moreno, J. C. Hybrid FES-robot cooperative control of ambulatory gait rehabilitation exoskeleton. J. Neuroeng. Rehabil. 11, 27 (2014).
Sanchez-Villamañan, M. D. C., Gonzalez-Vargas, J., Torricelli, D., Moreno, J. C. & Pons, J. L. Compliant lower limb exoskeletons: a comprehensive review on mechanical design principles. J. Neuroeng. Rehabil. 16, 55 (2019).
Kitatani, R. et al. Reduction in energy expenditure during walking using an automated stride assistance device in healthy young adults. Arch. Phys. Med. Rehabil. 95, 2128–2133 (2014).
Azocar, A. F. & Rouse, E. J. Characterization of open-loop impedance control and efficiency in wearable robots. IEEE Robot. Autom. Lett. 7, 4313–4320 (2022).
Allen, D. P. et al. Towards an ankle-foot orthosis powered by a dielectric elastomer actuator. Mechatronics 76, 102551 (2021).
Thalman, C. M. & Lee, H. Design and validation of a soft robotic ankle-foot orthosis (SR-AFO) exosuit for inversion and eversion ankle support. In IEEE International Conference on Robotics and Automation 1735–1741 (2020).
Thalman, C. M., Member, S., Hertzell, T., Debeurre, M. & Lee, H. The Multi-material Actuator for Variable Stiffness (MAVS): design, modeling, and characterization of a soft actuator for lateral ankle support. In IEEE/RSJ International Conference on Intelligent Robots and Systems 8694–8700 (2020).
Elevate ski exoskeleton. Roam Robotics https://www.roamrobotics.com/ski (2019).
Diller, S., Majidi, C. & Collins, S. H. A lightweight, low-power electroadhesive clutch and spring for exoskeleton actuation. In IEEE International Conference on Robotics and Automation 682–689 (IEEE, 2016).
Diller, S. B., Collins, S. H. & Majidi, C. The effects of electroadhesive clutch design parameters on performance characteristics. J. Intell. Mater. Syst. Struct. 29, 3804–3828 (2018).
Agarwal, P., Kuo, P.-H., Neptune, R. R. & Deshpande, A. D. A novel framework for virtual prototyping of rehabilitation exoskeletons. IEEE Int. Conf. Rehabil. Robot. 2013, 6650382 (2013).
Uchida, T. K. et al. Simulating ideal assistive devices to reduce the metabolic cost of running. PLoS ONE 11, e0163417 (2016).
Krebs, H. I. et al. HHS public access. Stroke 45, 200–204 (2014).
Kanzler, C. M., Lamers, I., Feys, P., Gassert, R. & Lambercy, O. Personalized prediction of rehabilitation outcomes in multiple sclerosis: a proof-of-concept using clinical data, digital health metrics, and machine learning. Med. Biol. Eng. Comput. 60, 249–261 (2022).
Mulroy, S., Gronley, J. A., Weiss, W., Newsam, C. & Perry, J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture 18, 114–125 (2003).
Filli, L. et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci. Rep. 8, 4984 (2018).
Mannini, A., Trojaniello, D., Cereatti, A. & Sabatini, A. A machine learning framework for gait classification using inertial sensors: application to elderly, post-stroke and Huntington’s disease patients. Sensors 16, 134 (2016).
Ferrante, S. et al. A biofeedback cycling training to improve locomotion: a case series study based on gait pattern classification of 153 chronic stroke patients. J. Neuroeng. Rehabil. 8, 47 (2011).
Martin, J. A. et al. Gauging force by tapping tendons. Nat. Commun. 9, 1592 (2018).
Jin, Y. et al. Soft sensing shirt for shoulder kinematics estimation. In IEEE International Conference on Robotics and Automation 4863–4869 (IEEE, 2020).
Slade, P., Kochenderfer, M. J., Delp, S. L. & Collins, S. H. Personalizing exoskeleton assistance while walking in the real world. Nature 610, 277–282 (2022).
Fickey, S. N., Browne, M. G. & Franz, J. R. Biomechanical effects of augmented ankle power output during human walking. J. Exp. Biol. 221, jeb182113 (2018).
Molinaro, D. D., Kang, I., Camargo, J., Gombolay, M. C. & Young, A. J. Subject-independent, biological hip moment estimation during multimodal overground ambulation using deep learning. IEEE Trans. Med. Robot. Bionics 4, 219–229 (2022).
Kanik, M. et al. Strain-programmable fiber-based artificial muscle. Science 365, 145–150 (2019).
Zhang, X. et al. Enhancing gait assistance control robustness of a hip exosuit by means of machine learning. IEEE Robot. Autom. Lett. 7, 7566–7573 (2022).
Medrano, R. L., Thomas, G. C. & Rouse, E. J. Can humans perceive the metabolic benefit provided by augmentative exoskeletons? J. Neuroeng. Rehabil. 19, 26 (2022).
Ingraham, K. A., Remy, C. D. & Rouse, E. J. The role of user preference in the customized control of robotic exoskeletons. Sci. Robot. 7, eabj3487 (2022).
George Hornby, T. Rethinking the tools in the toolbox. J. Neuroeng. Rehabil. 19, 61 (2022).
Labruyère, R. Robot-assisted gait training: more randomized controlled trials are needed! Or maybe not? J. Neuroeng. Rehabil. 19, 58 (2022).
Klingmann, I. et al. EUPATI and patients in medicines research and development: guidance for patient involvement in ethical review of clinical trials. Front. Med. 5, 251 (2018).
Torricelli, D. et al. Benchmarking wearable robots: challenges and recommendations from functional, user experience, and methodological perspectives. Front. Robot. AI 7, 561774 (2020).
Ármannsdóttir, A. L. et al. Assessing the involvement of users during development of lower limb wearable robotic exoskeletons: a survey study. Hum. Factors 62, 351–364 (2020).
Pinto-Fernandez, D. et al. Performance evaluation of lower limb exoskeletons: a systematic review. IEEE Trans. Neural Syst. Rehabil. Eng. 28, 1573–1583 (2020).
Koller, J. R., Gates, D. H., Ferris, D. P. & Remy, C. D. 'Body-in-the-loop’ optimization of assistive robotic devices: a validation study. Robot. Sci. Syst. https://doi.org/10.15607/RSS.2016.XII.007 (2016).
Siviy, C. et al. Offline assistance optimization of a soft exosuit for augmenting ankle power of stroke survivors during walking. IEEE Robot. Autom. Lett. 5, 828–835 (2020).
Dimyan, M. A. et al. Baseline predictors of response to repetitive task practice in chronic stroke. Neurorehabil. Neural Repair 36, 426–436 (2022).
Nesler, C., Thomas, G., Divekar, N., Rouse, E. J. & Gregg, R. D. Enhancing voluntary motion with modular, backdrivable, powered hip and knee orthoses. IEEE Robot. Autom. Lett. 7, 6155–6162 (2022).
Shorter, A. L. et al. Characterization and clinical implications of ankle impedance during walking in chronic stroke. Sci. Rep. 11, 16726 (2021).
Schweighofer, N. et al. Dissociating motor learning from recovery in exoskeleton training post-stroke. J. Neuroeng. Rehabil. 15, 89 (2018).
Reisman, D. S., Wityk, R., Silver, K. & Bastian, A. J. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130, 1861–1872 (2007).
Reisman, D. S., McLean, H., Keller, J., Danks, K. A. & Bastian, A. J. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil. Neural Repair 27, 460–468 (2013).
Reisman, D. S., Wityk, R., Silver, K. & Bastian, A. J. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil. Neural Repair 23, 735–744 (2009).
Day, K. A., Leech, K. A., Roemmich, R. T. & Bastian, A. J. Accelerating locomotor savings in learning: compressing four training days to one. J. Neurophysiol. 119, 2100–2113 (2018).
Dhawale, A. K., Smith, M. A. & Ölveczky, B. P. The role of variability in motor learning. Annu. Rev. Neurosci. 40, 479–498 (2017).
Marchal-Crespo, L., Lopez-Oloriz, J., Jaeger, L. & Riener, R. Optimizing learning of a locomotor task: amplifying errors as needed. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 5304–5307 (2014).
Sombric, C. J. & Torres-Oviedo, G. Augmenting propulsion demands during split-belt walking increases locomotor adaptation of asymmetric step lengths. J. Neuroeng. Rehabil. 17, 69 (2020).
Porciuncula, F. et al. Targeting paretic propulsion and walking speed with a soft robotic exosuit: a consideration-of-concept trial. Front. Neurorobot. 15, 689577 (2021).
Jonsdottir, J. et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabil. Neural Repair 24, 478–485 (2010).
Parker, C. J., Guerin, H., Buchanan, B. & Lewek, M. D. Targeted verbal cues can immediately alter gait following stroke. Top. Stroke Rehabil. 29, 382–391 (2022).
Miller, K. K., Porter, R. E., DeBaun-Sprague, E., Van Puymbroeck, M. & Schmid, A. A. Exercise after stroke: patient adherence and beliefs after discharge from rehabilitation. Top. Stroke Rehabil. 24, 142–148 (2016).
Leroux, A. Exercise training to improve motor performance in chronic stroke: effects of a community-based exercise program. Int. J. Rehabil. Res. 28, 17–23 (2005).
Macko, R. F. et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke. Stroke 36, 2206–2211 (2005).
Telehealth HOD P06191509 (APTA, 2019); https://www.apta.org/apta-and-you/leadership-and-governance/policies/telehealth
Dobkin, B. H. A Rehabilitation-Internet-of-Things in the home to augment motor skills and exercise training. Neurorehabil. Neural Repair 31, 217–227 (2017).
Sen-Gupta, E. et al. A pivotal study to validate the performance of a novel wearable sensor and system for biometric monitoring in clinical and remote environments. Digit. Biomark. 3, 1–13 (2019).
Ray, T. R. et al. Bio-integrated wearable systems: a comprehensive review. Chem. Rev. 119, 5461–5533 (2019).
Elery, T., Reznick, E., Shearin, S., McCain, K. & Gregg, R. D. Design and initial validation of a multiple degree-of-freedom joint for an ankle-foot orthosis. J. Med. Devices 16, MED-19-1201 (2022).
Fineman, R. A., McGrath, T. M., Kelty-Stephen, D. G., Abercromby, A. F. J. & Stirling, L. A. Objective metrics quantifying fit and performance in spacesuit assemblies. Aerosp. Med. Hum. Perform. 89, 985–995 (2018).
Tamez-Duque, J. et al. Real-time strap pressure sensor system for powered exoskeletons. Sensors 15, 4550–4563 (2015).
Acknowledgements
We thank L. Schumm and D. Orzel for help with the illustrations. This work was supported by the National Institutes of Health under award numbers BRG R01HD088619 and R01AG067394, and by the American Heart Association grant AHA 18TPA34170171. This work is based on studies supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE1144152, and by the National Science Foundation under Grant No. CMMI-1925085.
Author information
Authors and Affiliations
Contributions
C.S. and L.M.B. contributed equally to the writing and revision of the manuscript. C.S., L.M.B. and B.T.Q. drafted the section ‘Reduction of metabolic cost’. C.S., L.M.B. and F.P. drafted the section ‘In-clinic validation’. K.S. provided input to the ‘Component technology’ and ‘Outlook’ sections. C.J.W. and L.N.A. provided input on the overall manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Harvard University has entered into a licensing-and-collaboration agreement with ReWalk Robotics. C.J.W., C.S. and B.T.Q. are co-inventors on licensed patents and are paid consultants for ReWalk Robotics. L.N.A. is a paid consultant for MedRhythms. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Alessandra Pedrocchi, Gregory Sawicki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Tables
Supplementary Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siviy, C., Baker, L.M., Quinlivan, B.T. et al. Opportunities and challenges in the development of exoskeletons for locomotor assistance. Nat. Biomed. Eng 7, 456–472 (2023). https://doi.org/10.1038/s41551-022-00984-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00984-1
This article is cited by
-
Shaping high-performance wearable robots for human motor and sensory reconstruction and enhancement
Nature Communications (2024)
-
Terrain slope parameter recognition for exoskeleton robot in urban multi-terrain environments
Complex & Intelligent Systems (2024)
-
Ankle-targeted exosuit resistance increases paretic propulsion in people post-stroke
Journal of NeuroEngineering and Rehabilitation (2023)
-
A reprogrammable mechanical metamaterial with origami functional-group transformation and ring reconfiguration
Nature Communications (2023)
-
Hip and Knee Joint Kinematics Predict Quadriceps Hyperreflexia in People with Post-stroke Stiff-Knee Gait
Annals of Biomedical Engineering (2023)